Chemistry, at its core, is the science of transformation—taking what seems ordinary and uncovering the hidden choreography of atoms and bonds beneath the surface. Among the oldest and most enduring transformations in organic chemistry is the Ullmann reaction, discovered over a century ago. It is a copper-mediated reaction that allows chemists to link together carbon atoms or carbon with heteroatoms such as oxygen, nitrogen, or sulfur. This ability to form strong, versatile bonds has made the Ullmann reaction a cornerstone of modern organic synthesis, with applications ranging from pharmaceuticals to advanced materials.
Yet, despite its long history and wide use, the Ullmann reaction has always carried a mystery: how, exactly, does copper make this reaction happen?
For decades, chemists have debated the mechanism behind this transformation, particularly the oxidation states of copper as it cycles through the reaction. The prevailing idea was a simple back-and-forth exchange between copper(I) and copper(III). But there was a problem: copper(III) species are notoriously difficult to observe. The invisible steps in copper’s redox dance left the chemistry community divided, searching for definitive proof.
A Breakthrough in Understanding Copper
On September 22, a groundbreaking study published in Nature by Shen Qilong’s laboratory at the Shanghai Institute of Organic Chemistry, in collaboration with Professor K. N. Houk of UCLA, changed the conversation. Their work provided the clearest evidence yet that the Ullmann reaction is far more intricate than anyone had previously imagined.
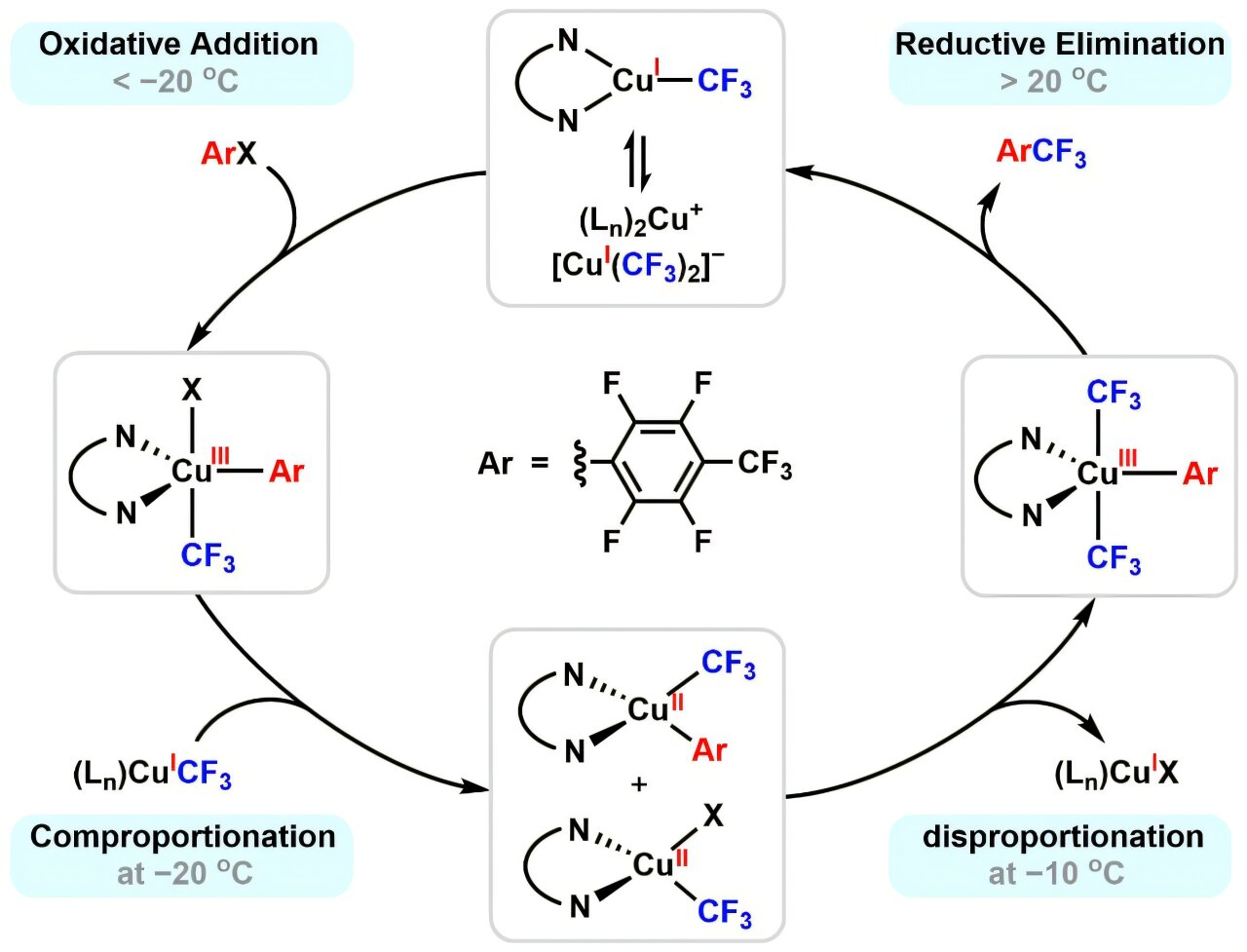
Instead of a simple Cu(I)/Cu(III) shuttle, the researchers discovered a more complex cycle: Cu(I)/Cu(III)/Cu(II)/Cu(III)/Cu(I). This finding not only resolves long-standing questions but also reshapes how chemists think about copper’s role in catalysis.
The discovery came through a clever experimental design. By carefully controlling the reaction temperature, the researchers slowed down the rapid chain of transformations, almost like hitting the pause button on a film, allowing them to capture the fleeting intermediates that usually escape notice.
Watching Copper’s Dance Step by Step
The experiments focused on reactions between copper(I) trifluoromethyl complexes and electron-deficient aryl iodides. At extremely low temperatures, around –20°C, the researchers observed the first act: oxidative addition and comproportionation processes that rapidly generated copper(II) species.
When the temperature was raised slightly, to –10°C, something remarkable happened. The copper(II) species did not stay stable. Instead, they underwent disproportionation, splitting into copper(III) and copper(I). This revealed a hidden transition step long suspected but never directly observed in such detail.
Finally, as the system warmed toward room temperature, the copper(III) intermediates released their energy through reductive elimination, completing the bond-forming step and regenerating copper(I). In this way, the catalytic cycle returned to its beginning, ready to repeat the process.
To ensure these fleeting copper states were real, the team used a combination of advanced spectroscopic methods: nuclear magnetic resonance (NMR) to probe structural changes, electron paramagnetic resonance (EPR) to detect unpaired electrons, and ultraviolet-visible (UV-Vis) spectroscopy to track the evolving copper species. Together, these techniques built a comprehensive picture of copper’s dynamic redox behavior.
More Than Just One Reaction
Perhaps the most exciting aspect of this work is that the mechanism was not unique to a single chemical system. The team extended their studies to other Ullmann-type reactions, including trifluoromethylation of various electron-deficient aryl iodides and the classic synthesis of biphenyl compounds. In each case, the same Cu(I)/Cu(III)/Cu(II)/Cu(III)/Cu(I) cycle appeared to govern the chemistry.
This strongly suggests that the newly uncovered pathway is not an exception but rather a general feature of Ullmann reactions. The implications are profound: chemists now have a new framework to guide the design and optimization of copper-catalyzed coupling reactions.
Why This Discovery Matters
At first glance, the mechanism of a century-old reaction might seem like an esoteric puzzle. But in chemistry, understanding mechanism is everything. It is the key to predicting outcomes, improving efficiency, and designing new reactions.
Copper is not just a tool of the Ullmann reaction—it is one of the most versatile metals in catalysis. From cross-coupling reactions in drug discovery to functionalization reactions in materials science, copper catalysis is everywhere. Knowing how copper switches between oxidation states opens doors to controlling these processes with greater precision, efficiency, and sustainability.
The discovery of the Cu(I)/Cu(III)/Cu(II)/Cu(III)/Cu(I) cycle also carries broader significance. It challenges the way chemists think about redox chemistry in transition-metal catalysis. Where older models emphasized simple two-state cycles, this new evidence shows that multi-step, dynamic cycles may be far more common than once believed.
A Glimpse Into Chemistry’s Future
This breakthrough is more than an academic triumph—it is a window into the future of chemical science. With new mechanistic clarity, researchers can better harness copper’s reactivity, designing greener reactions, minimizing waste, and opening possibilities for new pharmaceuticals, agrochemicals, and advanced functional materials.
Perhaps most importantly, this story illustrates the essence of scientific discovery. For decades, chemists debated copper’s hidden behavior. Some doubted that copper(III) could even exist in these reactions. But with persistence, creativity, and cutting-edge technology, a team of scientists froze the fleeting moment, revealed the truth, and deepened our understanding of one of chemistry’s most enduring mysteries.
In the end, the Ullmann reaction is not just about building bonds between atoms. It is about building bonds between questions and answers, between past knowledge and future innovation. And as this study shows, even a century-old reaction can still surprise us, reminding us that in science, the dance is never truly over—it only evolves into new steps waiting to be discovered.
More information: Yongrui Luo et al, Decoding the redox behaviour of copper in Ullmann-type coupling reactions, Nature (2025). DOI: 10.1038/s41586-025-09627-2