Every second of every day, an invisible miracle unfolds within your body—a process so essential, so fundamental, that life would not exist without it. This miracle is cellular respiration. It doesn’t take place in your lungs or mouth, despite the name. It happens deep within your cells, where tiny structures harness the chemical energy from food and convert it into something your body can actually use: adenosine triphosphate, or ATP.
Without cellular respiration, even the simplest action—blinking, thinking, beating your heart—would be impossible. It is the quiet engine that fuels life, the spark behind movement, sensation, and existence itself. Yet, for something so crucial, it often goes unnoticed.
What makes cellular respiration even more fascinating is that it comes in two primary flavors: aerobic and anaerobic. One demands oxygen, the other works without it. One is efficient and steady, the other is fast but limited. Both have roles to play in the biology of life, from the deepest ocean trenches to the human bloodstream. To truly understand how life functions, we must dive into the hidden world of respiration at the cellular level.
Energy: The Currency of Life
Every organism on Earth, from the smallest microbe to the largest whale, requires energy. This energy doesn’t just float freely—it must be harvested, processed, and stored. For animals like humans, energy comes primarily from food: carbohydrates, fats, and proteins that contain rich stores of chemical bonds.
Inside each of these molecules lies potential energy—energy that must be unlocked to be useful. Cellular respiration is the process by which cells break down these molecules, primarily glucose, to release energy in a usable form. The key product of this breakdown is ATP, often described as the energy currency of the cell.
ATP powers everything from nerve impulses to muscle contractions to DNA replication. Without it, life stops. So where does this ATP come from? The answer depends on the cell’s environment and evolutionary history—whether it has access to oxygen or not.
Glucose: The Star Fuel of Respiration
While cells can break down fats and proteins for energy, glucose is the preferred and most direct source. A simple sugar composed of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms (C₆H₁₂O₆), glucose is abundant, easily metabolized, and central to respiration.
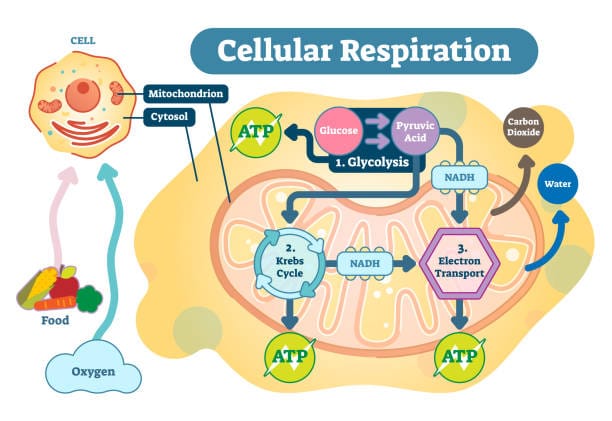
The story of glucose metabolism begins with glycolysis, the universal starting point of both aerobic and anaerobic respiration. This ancient biochemical pathway splits one molecule of glucose into two molecules of pyruvate, releasing a small amount of energy in the form of ATP and another molecule called NADH, which temporarily stores electrons.
Glycolysis occurs in the cytoplasm—the watery interior of cells—and does not require oxygen. In this way, it’s like the opening act of a performance that could go in one of two directions: toward oxygen-rich, high-yield aerobic respiration, or toward oxygen-poor, less efficient anaerobic respiration.
Aerobic Respiration: Oxygen’s Grand Design
When oxygen is available, cells embark on a highly efficient pathway known as aerobic respiration. This process takes place primarily in the mitochondria—organelles often dubbed the “powerhouses” of the cell. It’s here that the pyruvate from glycolysis is further processed, ultimately yielding large quantities of ATP.
Aerobic respiration consists of three main stages beyond glycolysis: the Krebs cycle (also known as the citric acid cycle), the electron transport chain, and oxidative phosphorylation. Each stage is intricately designed, involving dozens of enzymes and co-factors, all choreographed in exquisite detail.
In the mitochondria, pyruvate is converted into a molecule called acetyl-CoA, which enters the Krebs cycle. Here, it’s systematically dismantled, carbon atom by carbon atom, with the release of CO₂ as waste. But the real treasure is the transfer of high-energy electrons to NAD⁺ and FAD, creating NADH and FADH₂.
These electron carriers then journey to the electron transport chain, embedded in the inner mitochondrial membrane. Electrons are passed along a series of protein complexes, releasing energy at each step. This energy is used to pump protons across the membrane, creating a gradient—like water behind a dam.
Finally, these protons flow back through a channel called ATP synthase, spinning it like a turbine. This mechanical motion fuels the conversion of ADP into ATP. The final electron acceptor is oxygen, which combines with the electrons and hydrogen ions to form water.
The result? From a single molecule of glucose, aerobic respiration can generate up to 36–38 ATP molecules—a tremendous yield compared to the mere two ATPs from glycolysis alone. Oxygen, though invisible and often taken for granted, plays a starring role in this cellular symphony.
Anaerobic Respiration: Life Without Oxygen
But not all life has access to oxygen. In environments like deep soil, underwater sediments, or inside your digestive tract, oxygen may be scarce or entirely absent. And in times of high demand—like sprinting or lifting heavy weights—your muscle cells may outpace the oxygen supply. In these moments, cells switch to anaerobic respiration.
Anaerobic respiration also begins with glycolysis, producing pyruvate and 2 ATP. But instead of heading into the mitochondria, pyruvate is diverted into alternate pathways that regenerate NAD⁺, allowing glycolysis to continue. The result depends on the organism.
In human muscle cells, pyruvate is converted into lactic acid. This can accumulate and cause the familiar burning sensation during intense exertion. Although anaerobic respiration is inefficient in terms of ATP production, it’s rapid—a life-saving adaptation for short bursts of energy.
In microorganisms like yeast, pyruvate is converted into ethanol and carbon dioxide in a process known as alcoholic fermentation. This form of anaerobic respiration is responsible for bread rising and the creation of beer and wine—an elegant example of biology influencing culture and cuisine.
Other anaerobic organisms use alternate electron acceptors—nitrate, sulfate, or even iron—in place of oxygen. These adaptations reveal the incredible flexibility of life, shaped by billions of years of evolution to thrive in every conceivable niche on Earth.
The Evolution of Respiration
The origin of cellular respiration is deeply rooted in the history of life itself. The earliest life forms were anaerobic, emerging in a world without free oxygen. They relied on fermentation and other primitive pathways to extract energy.
Everything changed about 2.5 billion years ago during the Great Oxygenation Event, when photosynthetic bacteria began releasing oxygen as a byproduct. For many organisms, oxygen was toxic. But others adapted, developing internal mechanisms to not only tolerate it but harness its chemical power.
The emergence of aerobic respiration allowed organisms to produce vastly more energy, supporting greater complexity and paving the way for multicellular life. Mitochondria, the organelles responsible for aerobic respiration, are believed to have originated as free-living bacteria that were engulfed by early eukaryotic cells—a symbiotic merger that changed the course of evolution.
This partnership, known as endosymbiosis, gave rise to animals, plants, fungi, and protists. In a very real sense, you owe your existence to an ancient alliance between two cells—one that gave rise to the efficient, oxygen-driven energy system that powers your every thought and heartbeat.
When Things Go Wrong: Diseases of Respiration
Given its central importance, it’s not surprising that disruptions to cellular respiration can have devastating consequences. Inherited mitochondrial disorders can impair the body’s ability to generate ATP, leading to symptoms ranging from muscle weakness to neurological decline.
One such disease is Leigh syndrome, a rare genetic disorder that affects the mitochondria’s ability to produce energy, especially in the brain. Children with this condition often experience developmental delays, seizures, and early death.
Even common diseases, such as diabetes, stroke, or heart disease, can involve mitochondrial dysfunction. Cancer cells often shift their energy production toward glycolysis, even when oxygen is present—a phenomenon known as the Warburg effect. Understanding the nuances of cellular respiration is not just an academic exercise; it holds the key to treating and preventing a wide range of illnesses.
Exercise, Respiration, and Recovery
You’ve likely felt the effects of cellular respiration while exercising. During moderate aerobic activity, your body uses oxygen efficiently to generate ATP. You feel strong, steady, and energized.
But when the demand for ATP outpaces oxygen delivery, your body shifts toward anaerobic metabolism. This is when muscles produce lactic acid, leading to fatigue and soreness. The lactic acid is not a waste product, as once believed—it’s a fuel that can be recycled back into glucose by the liver through the Cori cycle.
Training increases the efficiency of your cardiovascular and respiratory systems, enhancing oxygen delivery and mitochondrial density in muscle cells. Over time, you rely less on anaerobic respiration and can sustain activity longer. Athletes don’t just train their muscles—they train their cells to breathe more efficiently.
The Universal Process
What’s truly astounding is that cellular respiration is not unique to humans. It is a shared feature of nearly all life on Earth. From bacteria in Arctic ice to fungi decomposing leaves in a rainforest, from wheat growing in a field to the neurons firing in your brain—cellular respiration unites us all in a single biochemical rhythm.
Despite the variations—different fuels, different pathways, different organisms—the core idea is the same: extracting usable energy from chemical bonds. In this way, cellular respiration is not just a process. It is the thread that weaves together all living things in a vast, interconnected tapestry.
A Delicate Balance in a Changing World
As the Earth warms and oxygen levels in certain environments fluctuate, understanding cellular respiration takes on new urgency. Coral reefs, for example, are sensitive to changes in oxygen and pH levels, which affect the respiration of both corals and the algae they depend on. Agricultural practices, climate change, and pollution can influence the oxygen balance in soil and water, affecting crop yields and food security.
Moreover, as scientists explore possibilities for life beyond Earth, respiration remains a key question. Could alien life use a form of anaerobic respiration? Would it rely on oxygen, or some other molecule entirely? Studying respiration on Earth helps us imagine what life might look like elsewhere—and what it truly means to be alive.
Final Thoughts: The Pulse Beneath the Surface
In the end, cellular respiration is more than a textbook diagram or a biochemical pathway. It is the heartbeat of life, the breath of cells, the flicker of energy that keeps us going from one moment to the next. It connects the microscopic to the macroscopic, the ancient to the modern, the personal to the planetary.
Every time you inhale, every bite of food you digest, every step you take—it all feeds into the quiet, relentless cycle of respiration. Aerobic or anaerobic, efficient or desperate, this process is as close as science gets to magic. And understanding it brings us closer to the wonder and fragility of life itself.