Beneath the surface of our skin, behind the symmetry of organs and tissues, and far beyond the perception of our naked eyes, lies a living world of extraordinary complexity. This is the world of cells—tiny, membrane-bound units of life that operate with astonishing precision and purpose. Yet, for all their complexity, cells are also profoundly dependent on their surroundings. They must constantly import and export materials to survive—taking in nutrients, signaling molecules, and even other cells, while also disposing of waste and releasing substances vital for the body.
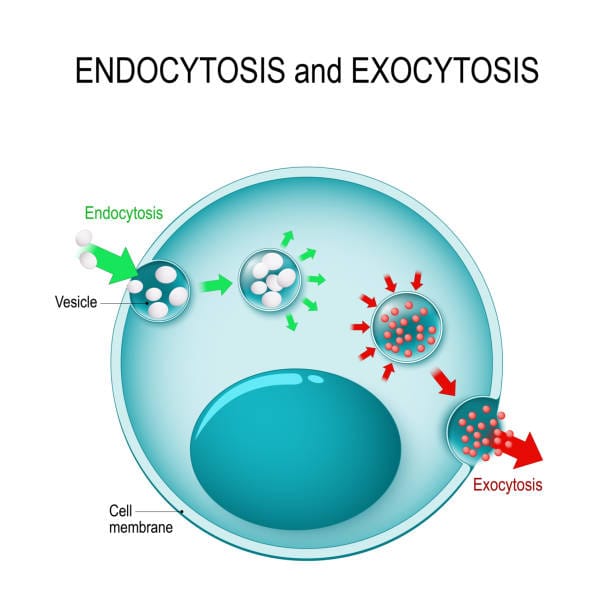
Among the most elegant and dynamic of these cellular processes are endocytosis and exocytosis—the mechanisms by which cells “eat” and “expel.” These processes are not just passive openings or leakages of the cell membrane. Rather, they are active, highly regulated dances involving intricate protein machinery, shifting membranes, and decisions that determine life and death at the cellular level.
To understand these processes is to grasp one of the most fundamental aspects of biology: the interface between a cell and its environment. And in that interface lies the key to immunity, communication, metabolism, development, and disease.
Life at the Cellular Frontier
The plasma membrane is more than just a boundary. It is the gatekeeper of life, a selectively permeable barrier composed of a fluid mosaic of lipids, proteins, and carbohydrates. Far from being static, the membrane is in constant motion, bending and reshaping itself to form vesicles—small, spherical sacs that transport cargo in and out of the cell.
Endocytosis and exocytosis are the processes by which vesicles are formed and used. They are not simple events but carefully choreographed processes involving dozens of molecular players. They ensure that cells can eat, drink, communicate, defend themselves, and even commit suicide when needed.
Without endocytosis, a neuron could not recycle its synaptic vesicles, a macrophage could not engulf a pathogen, and a cell could not respond to its hormonal signals. Without exocytosis, a pancreas could not secrete insulin, a neuron could not release neurotransmitters, and a developing embryo could not shape its body through cell migration.
Together, these twin processes form the basis of cellular interaction with the world. They are the cellular equivalents of breathing in and breathing out—deeply connected to survival and function.
Endocytosis: The Art of Cellular Ingestion
Endocytosis is the process by which cells internalize substances from their external environment. But this is not random or chaotic. Cells are choosy. They decide what to ingest, when to ingest it, and how to package it.
The most familiar form of endocytosis is phagocytosis—from the Greek meaning “cell eating.” This is the process by which cells engulf large particles, such as bacteria, apoptotic cells, or debris. It is a powerful and deliberate act of consumption, seen in immune cells like macrophages and neutrophils.
But there are subtler forms, too. Pinocytosis—or “cell drinking”—is a more general form of fluid uptake, in which the cell samples its environment by taking in small amounts of extracellular fluid. It’s less selective but equally important for balancing internal and external conditions.
And then there is receptor-mediated endocytosis, the most selective form of all. Here, receptors on the cell membrane recognize specific ligands—such as cholesterol, iron-carrying transferrin, or viruses—and trigger the formation of vesicles to bring these molecules into the cell. This form of endocytosis is like a molecular handshake, a mutually agreed-upon exchange that leads to internalization.
What makes these processes possible is the curvature and reshaping of the plasma membrane. Specialized proteins like clathrin and dynamin play central roles. Clathrin forms a cage-like structure that molds the membrane into a pit, while dynamin wraps around the neck of the pit and pinches it off, forming a vesicle. Once inside the cell, these vesicles are uncoated and directed to specific destinations—early endosomes, late endosomes, and ultimately lysosomes, where their cargo is digested or recycled.
The Cellular Stomach: Phagocytosis in Detail
Phagocytosis is a highly active and specialized process used primarily by immune cells to engulf large particles. When a pathogen such as a bacterium enters the body, it is quickly recognized by phagocytes. These cells extend their membrane around the invader using pseudopodia—temporary arm-like projections—and envelop it completely.
The engulfed particle becomes enclosed in a membrane-bound vesicle known as a phagosome. The phagosome then fuses with a lysosome, forming a phagolysosome, where the ingested material is degraded by acidic enzymes.
This process is not merely mechanical. It is deeply tied to immune recognition. Cells use pattern recognition receptors (PRRs) to identify pathogens based on molecular patterns common to microbes. Once a microbe is recognized and engulfed, parts of it are processed and presented on the cell surface, alerting the immune system and initiating a broader immune response.
In this way, phagocytosis is not just about eating—it’s about surveillance, defense, and communication. It connects the individual cell’s digestion to the immune system’s memory and learning.
Drinking from the Outside: Pinocytosis and Fluid Uptake
Pinocytosis is often overshadowed by the drama of phagocytosis, but it is no less vital. All cells use pinocytosis to some extent, continuously sampling the extracellular fluid to sense changes in nutrient levels, osmotic balance, and signaling molecules.
Unlike phagocytosis, pinocytosis does not rely on specific recognition. Instead, it is a continuous process in which small vesicles bud from the membrane and carry extracellular fluid into the cytoplasm.
In some cases, pinocytosis can be regulated by signaling pathways. For example, growth factors can stimulate cells to increase fluid uptake in preparation for proliferation. In others, it is used to internalize small soluble molecules that might otherwise diffuse too slowly.
The vesicles formed by pinocytosis often merge with early endosomes, where their contents can be sorted, sent to the Golgi apparatus, or degraded. This ongoing exchange keeps the cell in tune with its surroundings.
Receptor-Mediated Endocytosis: Precision at the Membrane
Perhaps the most elegant form of endocytosis is receptor-mediated. It exemplifies how cells combine recognition, signaling, and transport in a single operation.
In this process, specific molecules bind to receptors embedded in the plasma membrane. These receptors are often concentrated in areas coated with clathrin, a triskelion-shaped protein that assembles into a curved lattice. When enough receptors are occupied, the clathrin-coated pit invaginates and is pinched off into the cell, forming a coated vesicle.
Once inside, the vesicle sheds its clathrin coat and fuses with an early endosome. Here, the acidic environment causes the cargo to dissociate from its receptor. The receptors are recycled back to the membrane, while the cargo is processed or sent to lysosomes for degradation.
This process is vital for many physiological functions. Cholesterol uptake, for instance, depends on low-density lipoprotein (LDL) binding to LDL receptors and being internalized via clathrin-coated vesicles. Defects in this pathway can lead to atherosclerosis, emphasizing how a seemingly microscopic process can have massive implications for health.
Viruses, too, exploit this mechanism. Many viruses, including influenza and SARS-CoV-2, bind to specific receptors to gain entry into cells via endocytosis. Understanding these interactions has been crucial in developing vaccines and antiviral therapies.
From Inside Out: The Process of Exocytosis
While endocytosis brings material into the cell, exocytosis does the opposite—transporting cargo to the outside. This is how cells secrete hormones, neurotransmitters, digestive enzymes, and structural proteins like collagen.
Exocytosis begins with the formation of vesicles at the Golgi apparatus or from recycling endosomes. These vesicles are loaded with cargo and transported along the cytoskeleton to the plasma membrane. Once there, they dock, prime, and fuse with the membrane, releasing their contents into the extracellular space.
This process is regulated by a complex set of proteins known as SNAREs (soluble NSF attachment protein receptors). These proteins on the vesicle (v-SNAREs) and on the membrane (t-SNAREs) interact like a zipper, pulling the two membranes close enough to fuse.
Calcium ions often play a key role in triggering exocytosis. In neurons, for instance, the arrival of an action potential opens calcium channels, allowing Ca²⁺ to flood into the terminal. This sudden rise in calcium concentration causes synaptic vesicles to fuse with the membrane and release neurotransmitters into the synaptic cleft.
Exocytosis can be either constitutive—occurring continuously in all cells—or regulated—occurring only in response to specific signals. Secretory cells, like those in the pancreas or pituitary gland, use regulated exocytosis to control the release of substances precisely.
Recycling and Membrane Renewal
One of the lesser-known roles of endocytosis and exocytosis is their involvement in maintaining the plasma membrane itself. Every time a vesicle buds off from or fuses with the membrane, it adds or removes lipids and proteins. This contributes to membrane composition, fluidity, and signaling.
Cells use a delicate balance of endocytosis and exocytosis to recycle membrane components, renew their surface, and adjust to changes in size, shape, or environment. In neurons, for example, synaptic vesicles are continuously recycled after exocytosis to ensure sustained communication.
Moreover, cells undergoing division, migration, or morphogenesis rely on these processes to reshape themselves. Polarized cells—like those in the kidney or intestine—use targeted exocytosis and endocytosis to maintain distinct domains of their membrane.
Disorders in Cellular Trafficking
Given the complexity and precision of endocytosis and exocytosis, it’s no surprise that their malfunction can lead to disease. Mutations in genes encoding vesicle coat proteins, SNAREs, or motor proteins can cause neurological disorders, immune deficiencies, and metabolic syndromes.
For example, familial hypercholesterolemia is caused by mutations in the LDL receptor gene, preventing efficient endocytosis of cholesterol-rich lipoproteins. This leads to high blood cholesterol levels and early-onset heart disease.
Some cancers manipulate endocytosis to internalize growth factor receptors and avoid degradation, thereby sustaining uncontrolled growth signals. Others alter exocytosis to secrete enzymes that degrade tissue barriers, facilitating metastasis.
Even neurodegenerative diseases like Alzheimer’s and Parkinson’s show disruptions in vesicle trafficking, synaptic recycling, and membrane turnover. The buildup of misfolded proteins may result in part from failures in endocytic degradation.
The Evolutionary Origins of Membrane Trafficking
The ability to internalize and expel substances is not unique to animals. Single-celled eukaryotes like amoebae also use phagocytosis to engulf prey and pinocytosis to sample their environment. The evolution of endomembrane systems was a turning point in cellular evolution, giving rise to complex intracellular compartments and sophisticated signaling networks.
Endocytosis may have even played a role in the origin of eukaryotic cells themselves. According to the endosymbiotic theory, ancient ancestors of mitochondria and chloroplasts were engulfed by a host cell through a process resembling phagocytosis. These engulfed organisms were not digested but instead formed mutualistic relationships, eventually becoming organelles.
Thus, the processes of eating and expelling were not just life-sustaining—they were life-defining.
Endocytosis, Exocytosis, and the Future of Medicine
Our growing understanding of these processes has opened new frontiers in biotechnology and medicine. Drug delivery systems now harness receptor-mediated endocytosis to target specific tissues with nanocarriers. Vaccines use viral vectors that enter cells via endocytosis to deliver genetic instructions. Gene therapies are being designed to cross cellular membranes through engineered vesicles.
In neurobiology, manipulating exocytosis is key to understanding learning, memory, and mental health. Disorders like schizophrenia, autism, and epilepsy are increasingly viewed through the lens of synaptic vesicle dynamics.
Cancer therapies are now exploring how to block or exploit exocytic pathways to limit tumor spread or enhance immune targeting. And regenerative medicine relies on exosomes—tiny vesicles released by cells—to mediate healing and tissue repair.
What began as the study of cellular ingestion and secretion has become a foundation for some of the most exciting and promising advances in modern science.
Conclusion: The Secret Choreography of Life
Endocytosis and exocytosis are not just cellular logistics. They are fundamental expressions of life—mechanisms by which cells know their world, share with it, defend against it, and adapt to its changes.
They are the breath of the cell, the tide of molecules that rises and falls with every signal, every challenge, every need.
To watch these processes under a microscope is to witness a kind of dance—a dynamic, responsive choreography that reflects billions of years of evolution. Every vesicle that forms, every membrane that bends, every signal that triggers release, speaks to a deeper truth: that life, at its core, is interaction.
It is not enough for a cell to exist. It must connect. And in those connections—at the shimmering boundary between inside and outside—life reveals its extraordinary grace.