Every movement you make, every breath you take, every thought that flickers across your brain happens because of an invisible molecule working tirelessly inside you. This molecule doesn’t get headlines or glory, yet it is the biochemical currency of life. Its name is ATP—adenosine triphosphate—and it is the unsung hero powering every cell in your body and in every other living organism on Earth.
At first glance, ATP looks like just another chemical compound. But don’t be fooled by its modest size or unassuming structure. ATP is not just essential; it is the molecule that makes life possible. From single-celled bacteria swimming through puddles to the complex neuronal firing of the human brain, ATP is the universal energy provider. Without it, life would grind to an immediate halt.
To understand life at its most fundamental level, we must journey deep inside the cell, into the microcosm where molecular machines work with breathtaking precision. There, among the mitochondria, enzymes, and membranes, ATP reigns supreme—not by force, but by energy.
ATP: A Molecular Power Unit
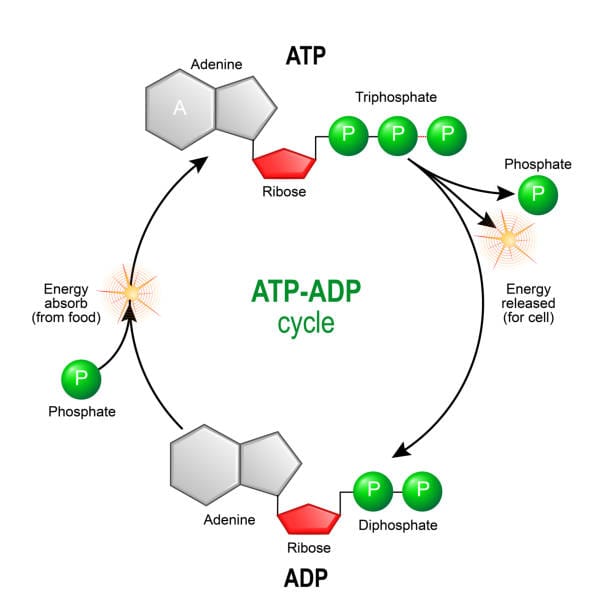
ATP stands for adenosine triphosphate, and its structure, though compact, is full of potential. It consists of three key parts: an adenine base (a nitrogen-containing molecule), a ribose sugar (a five-carbon sugar), and three phosphate groups lined up in a row. It’s these phosphate groups that give ATP its explosive character.
Each of the phosphate bonds, particularly the bond between the second and third phosphate group, is a high-energy bond. When ATP is broken down—typically through a reaction called hydrolysis—it loses one of its phosphate groups and releases a burst of energy. The result is ADP (adenosine diphosphate) and a free inorganic phosphate (Pi), and in the process, energy is unleashed that fuels the work of the cell.
But why is the bond so high in energy? The key lies in the electrostatic repulsion among the negatively charged phosphate groups. Like magnets pushing each other apart, the phosphates are under tension, and when the terminal bond is broken, the release is akin to a spring uncoiling. The energy doesn’t come from the bond itself, but from the chemical environment shifting from a high-energy, unstable state to a lower, more stable one.
In essence, ATP is like a fully charged battery, ready to discharge energy at a moment’s notice.
The Constant Cycle of Synthesis and Use
Incredibly, a single human cell uses and regenerates millions of ATP molecules per second. Your entire body turns over its own weight in ATP every day. The molecule isn’t stored in vast reserves—it’s recycled continuously. As soon as ATP is used, it is almost immediately reassembled in processes that require energy input, most notably cellular respiration.
The regeneration of ATP from ADP and Pi is driven by energy extracted from nutrients. In aerobic organisms, this takes place in the mitochondria—the cell’s powerhouses—through a process known as oxidative phosphorylation. This process couples the oxidation of glucose or other fuel molecules with the synthesis of ATP. In anaerobic organisms or low-oxygen conditions, ATP can also be generated through fermentation, though much less efficiently.
ATP is not just a passive carrier of energy; it’s part of a dynamic cycle of give-and-take, constantly shifting between high-energy (ATP) and low-energy (ADP) states. This balance is what keeps the cellular economy running.
Mitochondria: The ATP Factories
To understand how ATP is produced, we must dive into the mitochondria—specialized organelles nestled within most of your cells. They are oval-shaped, double-membraned structures that play a critical role in converting the energy locked in food molecules into ATP.
Inside the mitochondrion, cellular respiration unfolds in several carefully choreographed steps. Glycolysis, the first step, occurs in the cytoplasm and breaks down glucose into pyruvate, producing a small amount of ATP directly. The pyruvate then enters the mitochondrion, where it undergoes further processing in the Krebs cycle (also called the citric acid cycle), generating electron-rich molecules like NADH and FADH₂.
These molecules then pass their high-energy electrons to the electron transport chain embedded in the inner mitochondrial membrane. As electrons flow down this chain, their energy is used to pump protons (H⁺ ions) across the membrane, creating a proton gradient—a kind of cellular dam holding back a flood of potential energy.
The final act is chemiosmosis. Protons flow back into the mitochondrial matrix through ATP synthase, a molecular turbine that harnesses this flow to add a phosphate group to ADP, creating ATP. It’s an astonishingly efficient process, one that transforms a glucose molecule into about 30-32 ATP molecules under ideal conditions.
The elegance of this system is staggering. Every heartbeat, every muscle contraction, every moment of thought is powered by the ceaseless work of these minuscule engines.
The Universal Currency of Life
What makes ATP so remarkable is its universality. It is used by every known form of life—from archaea in scalding hydrothermal vents to humans walking through a park. The fact that evolution has conserved this molecule across billions of years suggests just how effective ATP is as an energy currency.
This universality also makes ATP a kind of molecular lingua franca—a common language spoken by cells regardless of their species. Whether a cell is building proteins, replicating DNA, repairing damage, transporting molecules, or sending signals, it uses ATP to pay for the work.
ATP binds to enzymes and changes their shape, enabling them to catalyze reactions. It activates motor proteins like kinesin and dynein that walk along microtubules, dragging cellular cargo to where it’s needed. It fuels pumps in cell membranes that maintain the delicate balance of ions necessary for nerve impulses and muscle contractions. It even drives the folding and unfolding of proteins, ensuring they achieve their proper functional shape.
In every cell, at every moment, ATP is being spent, earned, and spent again in a biochemical economy more dynamic and responsive than any stock market.
Muscle Contraction: ATP in Action
Nowhere is ATP’s role more visible—or more dramatic—than in muscle contraction. Every time you blink, lift your arm, or take a step, ATP is hard at work converting chemical energy into mechanical movement.
Within muscle cells, proteins called actin and myosin slide past each other to shorten the muscle fiber. This sliding mechanism is powered by ATP. Myosin heads bind to actin, forming cross-bridges, and then pivot—pulling the actin filaments along. ATP then binds to the myosin head, causing it to release from actin and reset for another cycle.
Without ATP, myosin cannot detach from actin. This is why, after death, muscles become stiff—a condition known as rigor mortis—because ATP production ceases and cross-bridges remain locked.
This beautifully orchestrated dance of proteins, fueled by ATP, is what allows your heart to beat and your body to move.
ATP and Nerve Impulses
ATP also powers the most sophisticated electrical system in the human body: the nervous system. Neurons communicate via electrical impulses, which depend on the movement of ions like sodium (Na⁺) and potassium (K⁺) across the cell membrane.
Maintaining the difference in ion concentrations inside and outside the neuron requires constant energy. Enter the sodium-potassium pump, an enzyme that actively transports three sodium ions out and two potassium ions in, against their concentration gradients. This pump consumes a large share of the cell’s ATP budget.
When a neuron fires, it opens ion channels, allowing sodium and potassium to rush in and out, creating a wave of electrical activity. Afterward, the pumps must restore the original ion gradients so the neuron can fire again. Without ATP, the system would collapse, and the brain would go dark.
Your every thought, memory, sensation, and decision rides on waves of ATP-fueled electrochemical activity.
DNA Replication and Protein Synthesis
Cells not only live—they grow, divide, and adapt. These processes require complex machinery, all of which are powered by ATP.
During DNA replication, enzymes unwind the double helix, synthesize complementary strands, and correct errors. Each of these steps consumes energy, much of it provided by ATP. Helicase enzymes use ATP to separate the DNA strands, while DNA ligase uses it to seal breaks in the sugar-phosphate backbone.
Protein synthesis, too, is a costly affair in terms of ATP. Ribosomes, the molecular factories that build proteins, require ATP to initiate and elongate the amino acid chain. Amino acids must be “charged” with ATP before they can be added to a growing protein, and multiple ATP molecules are spent for every amino acid incorporated.
In essence, ATP makes the very blueprint of life readable and executable.
Cell Signaling and Communication
Cells must also talk to each other, coordinating everything from immune responses to growth and development. Many of these signals are transmitted using molecules that depend on ATP.
One example is cyclic AMP (cAMP), a derivative of ATP that acts as a second messenger in numerous signaling pathways. When a hormone or neurotransmitter binds to a receptor on the cell surface, it can trigger the conversion of ATP into cAMP inside the cell, which then activates protein kinases that alter cell behavior.
In this way, ATP is not just a fuel but also a message—a switch that turns processes on or off and a bridge between the outside world and the cell’s interior.
The Fragility of the ATP System
Because life depends so completely on ATP, anything that disrupts its production can be catastrophic. Mitochondrial diseases, which impair ATP synthesis, can affect the brain, muscles, heart, and other energy-hungry tissues. Conditions like stroke or heart attack, which block oxygen supply, stop ATP production within minutes—leading to cell death.
Even poisons like cyanide work by targeting the electron transport chain, halting ATP production and suffocating the cell’s ability to function.
These vulnerabilities highlight just how finely balanced and essential ATP production is. Life hangs on this molecular thread.
The Evolutionary Perspective
How did such a perfect energy system come to be?
ATP likely emerged very early in the history of life, perhaps more than 3.5 billion years ago. Primitive cells might have used simpler molecules like pyrophosphate, but ATP eventually took over because of its stability, versatility, and compatibility with emerging enzymatic systems.
As evolution progressed, ATP-based metabolism became more complex and efficient. The emergence of mitochondria—likely through endosymbiosis, where one cell engulfed another—revolutionized energy production and enabled the rise of multicellular life.
Every innovation in biological evolution—from the first twitching protozoan to the flowering of consciousness—has been supported by ATP’s reliable energy transfers.
Beyond Earth: ATP and the Search for Life
Astrobiologists, scientists who study the potential for life beyond Earth, often consider ATP (or ATP-like molecules) as a marker of life. If extraterrestrial life exists, it will need some form of energy currency—and ATP is a compelling candidate.
That said, ATP may not be the only possibility. In hypothetical alien biochemistries, other molecules might serve similar roles. But here on Earth, where life has had billions of years to optimize itself, ATP remains king.
Its ubiquity and efficiency make it a potential biosignature—a molecule whose detection might one day indicate life on distant worlds.
Conclusion: The Fire Within
ATP is often described as life’s “energy currency,” but that phrase hardly does justice to its importance. It is not merely a medium of exchange. It is the fire that keeps the cell alive, the pulse beneath every heartbeat, the surge behind every thought. It is energy made tangible, stored in a single, elegant molecule.
In every living cell, ATP acts as both servant and sovereign—obedient to the enzymes that command it, yet holding the very power that animates life. It is both the spark and the fuel, both the match and the flame.
When we marvel at the complexity of the human brain, the grace of an athlete, the speed of a hummingbird, or the resilience of life itself, we are witnessing ATP in motion—a molecule that, though microscopic and unseen, holds up the entire edifice of biology.
And so, buried deep within the quiet architecture of life, ATP never sleeps. It powers the birth of cells and the dying of stars. It is the whisper of breath and the roar of movement. It is life’s most reliable engine—and its most sacred secret.