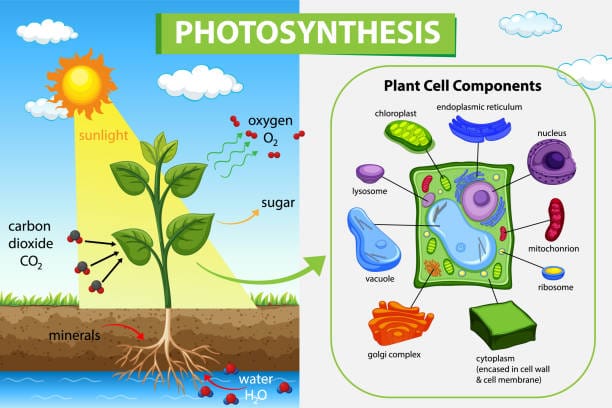
Beneath the green canopy of trees, within blades of grass, and inside the velvety folds of every leaf on Earth, an ancient process unfolds with quiet brilliance. It is called photosynthesis—the astonishing cellular ballet that captures sunlight and converts it into chemical energy, literally turning light into life. Plants perform this miracle every second of every day, not only to survive, but to sustain nearly all life on Earth. Without photosynthesis, there would be no forests, no food chains, and no breathable atmosphere.
What appears to be a simple greening of the world is actually a highly orchestrated sequence of molecular events. At the microscopic scale of chloroplasts—tiny organelles within plant cells—an energetic drama plays out. Molecules dance, electrons leap, water splits, and sugar is born. In this alchemy of sunlight, carbon dioxide, and water, energy is captured and life is nourished.
Understanding photosynthesis is not only a journey into the heart of plant biology; it is a doorway into the deep structure of ecology, climate, and even the human future. It is, quite simply, the most important chemical process on the planet.
Chloroplasts: The Powerhouse of the Plant Cell
Photosynthesis takes place inside specialized structures within plant cells called chloroplasts. These organelles are descendants of ancient cyanobacteria, once free-living photosynthetic microbes that were engulfed by ancestral plant cells in a symbiotic embrace over a billion years ago. This endosymbiotic relationship was so successful that chloroplasts became a permanent fixture, and their descendants exist in every leaf today.
Chloroplasts are enclosed by a double membrane and filled with a dense fluid called stroma. Within the stroma are stacks of flattened membrane sacs called thylakoids, which contain the pigment chlorophyll. These thylakoids are where the light reactions of photosynthesis begin, bathed in solar radiation like tiny solar panels collecting light.
The green hue of leaves comes from chlorophyll, a pigment exquisitely tuned to absorb light from the blue and red portions of the spectrum. Green light, being poorly absorbed, is reflected—giving plants their verdant glow. But that reflected light is merely a byproduct. What matters is what gets absorbed, and what happens next inside these emerald factories.
The Two-Stage Architecture of Photosynthesis
Photosynthesis unfolds in two broad stages, each with its own cast of molecules, enzymes, and energetic transformations. First come the light-dependent reactions, where the energy of sunlight is harvested and stored in energy carriers. These reactions occur in the thylakoid membranes and require light to function.
The second stage, called the light-independent reactions (also known as the Calvin cycle), occurs in the stroma and does not require light directly. Instead, it uses the energy captured during the first stage to synthesize sugars from carbon dioxide. Together, these two stages form a tightly coupled metabolic engine that drives the conversion of light energy into stable chemical bonds.
Let’s now walk through this process step by step—from the very moment sunlight touches a leaf, to the final creation of the sugars that feed the world.
Capturing the Light: The Photon’s First Step
Photosynthesis begins when a photon—a particle of light—strikes a chlorophyll molecule embedded in the thylakoid membrane. This event occurs in large protein complexes known as photosystems, primarily Photosystem II and Photosystem I. Each photosystem is like a molecular antenna surrounded by accessory pigments that funnel light energy toward a reaction center.
When a photon excites an electron in chlorophyll, the electron is boosted to a higher energy state and is ejected from the molecule. This energized electron doesn’t wander aimlessly; it is swiftly captured by a nearby molecule and passed through a chain of proteins called the electron transport chain.
This chain is not just a conduit—it is an energy staircase. As the electron steps down from one protein to another, it releases energy that is used to pump hydrogen ions (protons) across the thylakoid membrane, creating an electrochemical gradient.
Water Splitting: The Oxygen Revelation
But electrons are not infinite. For the system to keep functioning, the ejected electron must be replaced. This replacement comes from the splitting of water molecules—a reaction so elegant and vital that it shapes the atmosphere itself.
In the oxygen-evolving complex of Photosystem II, water molecules are split into electrons, protons, and molecular oxygen:
2H2O→4H++4e−+O22H_2O \rightarrow 4H^+ + 4e^- + O_2
The electrons replenish those lost by chlorophyll. The protons contribute to the electrochemical gradient. And the oxygen—released as a byproduct—escapes into the air, sustaining every breathing organism on Earth. This is the only known biological process that produces oxygen gas, and it has done so for billions of years, transforming Earth’s primordial atmosphere and paving the way for animal life.
ATP and NADPH: The Energy Currency of Life
As the electron continues down the transport chain, it reaches Photosystem I, where it receives another boost of energy from light. It is then transferred to the molecule NADP⁺, reducing it to NADPH, a high-energy electron carrier.
Meanwhile, the accumulated protons inside the thylakoid lumen create a proton motive force. Like water behind a dam, this pressure drives protons back across the membrane through a protein complex called ATP synthase. This enzyme acts like a molecular turbine, spinning as protons flow through it, catalyzing the formation of ATP from ADP and inorganic phosphate.
The light reactions thus produce two critical energy molecules: ATP and NADPH. They are not the final products of photosynthesis, but they serve as portable batteries—charged and ready to fuel the next stage: carbon fixation.
The Calvin Cycle: From Air to Sugar
The Calvin cycle occurs in the stroma, where carbon dioxide from the atmosphere is incorporated into organic molecules. This process is powered by the ATP and NADPH generated in the light reactions.
It begins with a remarkable enzyme called Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase), one of the most abundant enzymes on Earth. Rubisco attaches CO₂ to a five-carbon sugar called ribulose bisphosphate (RuBP), producing an unstable six-carbon compound that quickly splits into two three-carbon molecules called 3-phosphoglycerate (3-PGA).
Through a series of reactions involving ATP and NADPH, these molecules are transformed into glyceraldehyde-3-phosphate (G3P), the building block of glucose and other carbohydrates. Some G3P molecules exit the cycle and are used to synthesize sugars, starch, and cellulose. Others are recycled to regenerate RuBP, allowing the cycle to continue.
The Calvin cycle is slow and inefficient by some standards, but it is relentless. It operates billions of times a second across the biosphere, quietly weaving air into structure, energy, and life.
What Plants Do With the Sugar They Make
The sugars produced in photosynthesis are the lifeblood of the plant. Glucose can be used immediately in cellular respiration to provide energy, or it can be polymerized into starch for long-term storage. Some is converted into cellulose, the fibrous material that makes up the plant’s cell walls. Sugars also serve as precursors for amino acids, lipids, and nucleic acids.
But plants don’t keep all their bounty. Through their roots, fruits, and leaves, they feed ecosystems. Herbivores graze on leaves. Pollinators sip nectar. Decomposers break down plant litter. Even carnivores, at the end of long food chains, rely ultimately on plant photosynthesis. It is the primary input of energy into the biosphere, the very foundation of the food web.
Climate, Carbon, and Photosynthesis
Photosynthesis plays a central role in Earth’s carbon cycle. Every year, terrestrial plants remove an estimated 120 gigatons of carbon dioxide from the atmosphere through photosynthesis, offsetting much of the CO₂ released by respiration, decay, and human activity.
But with the rise of fossil fuel combustion, deforestation, and climate change, this balance is shifting. As CO₂ levels rise, photosynthesis may temporarily accelerate—a phenomenon known as CO₂ fertilization—but this effect is limited by water, nutrients, and temperature. Prolonged heatwaves and droughts can cripple plant function, reduce growth, and even reverse forests into carbon sources rather than sinks.
Understanding photosynthesis, therefore, is not just a matter of biology—it is a matter of planetary health. It is the mechanism by which we hope to draw down carbon, regenerate forests, and stabilize the climate.
Photosynthesis in Other Organisms
While we often associate photosynthesis with green plants, the process is older and more diverse than that. Algae, cyanobacteria, and certain protists also perform photosynthesis, and collectively they contribute more than half of Earth’s oxygen and fixed carbon.
Marine phytoplankton, though microscopic, are planetary heavyweights. These floating photosynthesizers form the base of oceanic food chains and regulate atmospheric gases. Rainforests may be the lungs of the land, but the oceans are the lungs of the planet.
Some organisms, like purple sulfur bacteria, perform a form of photosynthesis that doesn’t produce oxygen. These anoxygenic pathways hint at earlier versions of the process, relics from a time before cyanobacteria reshaped the world.
Human Ingenuity Inspired by Photosynthesis
Scientists and engineers are now trying to mimic photosynthesis to solve human problems. Artificial photosynthesis is a field that seeks to replicate the light-driven reactions of plants to create clean fuels, such as hydrogen or methanol, using sunlight and water. These synthetic systems aim to close the loop between energy production and carbon fixation, potentially revolutionizing renewable energy.
Meanwhile, biotechnologists are enhancing natural photosynthesis through genetic modification, aiming to create crops that grow faster, use less water, or capture more CO₂. These innovations are vital in a world facing food insecurity and ecological stress.
Even space exploration hinges on photosynthesis. As we imagine human missions to Mars or lunar colonies, closed-loop systems based on plant photosynthesis become essential for recycling air, water, and nutrients.
The Spiritual Beauty of Photosynthesis
Beyond its scientific significance, photosynthesis is a source of wonder. It is a poem written in the language of molecules, a fusion of physics and life that bridges the cosmos and the cell. That light, emitted from a star 150 million kilometers away, can be caught by a chlorophyll molecule and transformed into sugar is nothing short of miraculous.
To watch a forest bloom, a field ripple with green, or a sprout push through soil is to witness the quiet power of photosynthesis. It is a reminder that life is not only a struggle for survival but a choreography of light and chemistry. It tells us that the sun does not merely shine upon life—it is life, through the agency of photosynthesis.
Conclusion: A Process That Sustains the World
Photosynthesis is more than a biological process. It is the foundation of life on Earth, the engine of ecosystems, the filter of our air, the root of our food, and the guardian of our climate. It is ancient and ever-renewing, fragile and robust, simple in principle and breathtaking in detail.
In each leaf, each blade of grass, and every algae cell drifting in the sea, sunlight is captured, water is split, and carbon is fixed—all with a precision and elegance that continues to inspire scientists and poets alike.
Understanding photosynthesis is to glimpse the secret that plants have kept for over 3 billion years: that life can feed on light, that energy can be stored in sugars, and that the invisible work of cells sustains the visible world we inhabit.