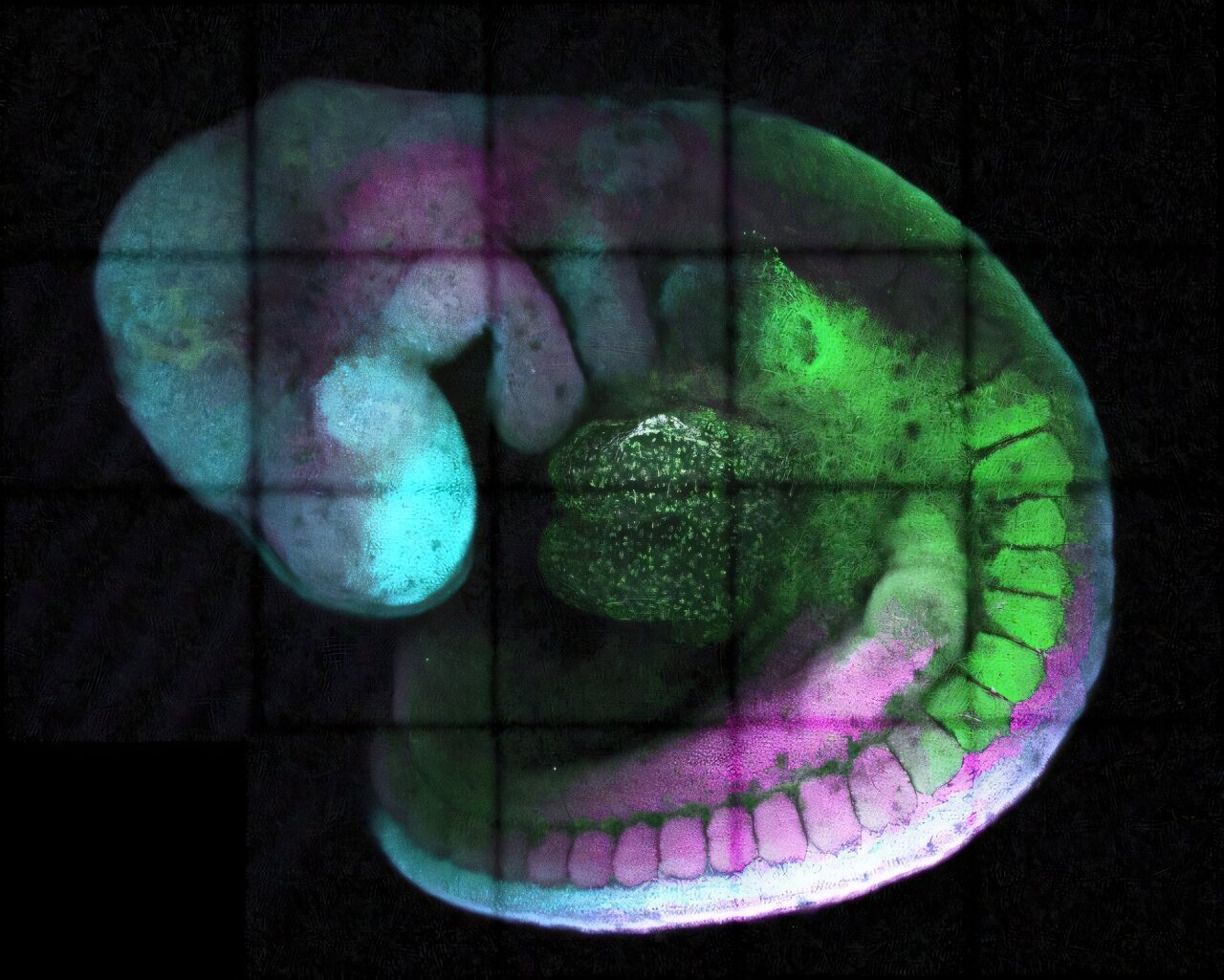
Inside every cell of your body lies the same instruction manual: your DNA. Whether you’re looking at the cells that form your beating heart, your thinking brain, or the skin at the tip of your nose, the DNA inside is identical. But clearly, not every cell becomes the same. The question is: why?
This ancient riddle of biology—how cells with identical DNA become wildly different—just took a groundbreaking turn.
A team of scientists from the MRC Laboratory of Medical Sciences (LMS) in the UK has uncovered a previously unknown way that genes are switched “on” and “off” during early embryonic development. Their findings, published in the journal Developmental Cell, don’t just explain how our bodies form in the womb—they may open new doors to treating diseases that stem from faulty gene control.
Finding the Hidden Hand Behind Cell Fate
The research, led by Dr. Irène Amblard and Dr. Vicki Metzis of the LMS’s Development and Transcriptional Control group, focused on a critical player in development: a gene called Cdx2.
Cdx2 isn’t just any gene—it’s a master regulator, particularly important in the formation of the spinal cord. But it needs to be activated at just the right time and for just the right amount of time for things to go smoothly. Too long, and it can wreak havoc. Too short, and important structures might never form. This delicate window of activity raised a question: how does the cell know when to stop?
What they discovered wasn’t a traditional gene or a powerful “on/off” switch like previously known enhancers or silencers. It was something more subtle, more refined—a piece of DNA they named an “attenuator.”
The Birth of a Biological Dimmer Switch
Attenuators, as this team found, don’t simply toggle genes on or off. Instead, they adjust the strength and timing of gene activity. Think of them as a dimmer switch, finely tuning how brightly the gene shines. In the case of Cdx2, this dimmer made sure the gene was expressed only briefly and precisely where needed in the embryo.
“When we altered this DNA element,” said Dr. Metzis, “we were able to tweak how strongly or how long Cdx2 was turned on—like adjusting the brightness of a light bulb rather than flipping a switch.”
By disrupting this element in mouse embryos, they confirmed just how vital it was. Without it, the embryo’s development didn’t go as planned—certain body regions failed to form properly, reinforcing the importance of this tiny piece of DNA in building complex life.
Why This Changes Everything
We’ve long known that genes don’t act in isolation. They’re part of a symphony, a vast orchestra of signals that play out during development and throughout life. For decades, scientists have studied the “instruments” that blast these signals: enhancers to ramp up gene activity, silencers to suppress it.
But attenuators add a new layer—nuance. They don’t scream or silence; they whisper. And in the finely tuned world of biology, those whispers matter.
This discovery not only helps us understand how embryos develop, but it opens a new door in medicine: programmable gene expression. If we can harness these genetic dimmers, scientists might one day fine-tune gene activity to correct diseases, without needing to rewrite the genetic code entirely.
The Silent Majority of DNA Speaks
The research also adds to a growing realization: most of our genome doesn’t code for proteins. For years, that non-coding DNA was dismissed as “junk.” But it’s becoming increasingly clear that this so-called junk holds the keys to how our genes are used—like the conductor behind the scenes of the genomic orchestra.
“This discovery adds to the idea that there are many different kinds of regulatory elements in our DNA that we still don’t fully understand,” said Dr. Metzis. “We’re excited because previous research suggests the genome contains many such elements that help finely tune gene expression—but they’ve been incredibly hard to find.”
The identification of attenuators provides researchers with a new blueprint to explore the dark matter of the genome—a realm that could hold the secrets to treating everything from developmental disorders to cancer, and even aging-related decline.
A Future of Fine-Tuned Medicine
Imagine treating a disease not by replacing a faulty gene, but by adjusting its expression like turning a volume knob. Not turning it off completely, not amplifying it blindly, but precisely modulating it in the affected tissue at just the right time. This is the future that discoveries like this point toward.
Rather than editing the genome with a sledgehammer, we may soon use a scalpel—a softer, more sophisticated approach that respects the complexity of biology.
Such a vision is still years away, but the path is becoming clearer.
The Code Within the Code
The study is a collaboration between several LMS research groups, including Chromatin and Development and Computational Regulatory Genomics. Together, they combine cutting-edge laboratory techniques with advanced computational models, enabling them to peer into the genome at an unprecedented level of detail.
Their work offers both a profound insight into the earliest chapters of life and a glimpse of how future medicine could be written—not just in genes, but in how we control them.
It’s a reminder that DNA isn’t just a static instruction book. It’s a living, dynamic system—more like software than hardware. And for the first time, we’re beginning to understand the hidden lines of code that make us who we are.
Reference: A dual enhancer-attenuator element ensures transient Cdx2 expression during mouse posterior body formation, Developmental Cell (2025). DOI: 10.1016/j.devcel.2025.06.006. www.cell.com/developmental-cel … 1534-5807(25)00361-2