In a quiet laboratory at the Institute for Bioengineering of Catalonia (IBEC), something extraordinary is happening—something that blurs the line between living and lifelike. Here, in the stillness of a microscope’s gaze, artificial cells—tiny, engineered bubbles—are beginning to move.
Not randomly, not aimlessly, but with purpose. Like hungry bacteria sniffing out a sugar trail, these synthetic entities are capable of chemotaxis: they sense, respond, and migrate toward specific chemical signals in their environment.
For the first time, scientists have engineered an artificial cell with the ability to follow chemical gradients, mimicking one of nature’s most fundamental biological strategies. Published in Science Advances, this work not only provides a blueprint for synthetic life, but it also opens a window into life’s earliest steps—when chemistry began to behave like biology.
The Quiet Intelligence of Chemotaxis
Before muscles, before brains, before nervous systems could steer a creature through its environment, life had already found a way to move with intention. This behavior is called chemotaxis—the ability to migrate toward favorable conditions, like food, or away from danger, like toxins.
“Bacteria use it to locate nutrients. White blood cells follow it to track infection. Even sperm navigate toward the egg using chemotaxis,” says Bárbara Borges Fernandes, lead author of the study and a researcher in the Molecular Bionics group at IBEC.
It is not just movement; it is direction, decision, and in a primitive sense, choice. Until now, such behaviors were thought to rely on sophisticated cellular machinery—molecular motors, receptors, cytoskeletal reorganization. But what if complexity wasn’t required? What if the very principles of directed life could emerge from something far simpler?
That’s exactly what the IBEC team set out to prove.
The Minimal Cell: Simple Form, Profound Function
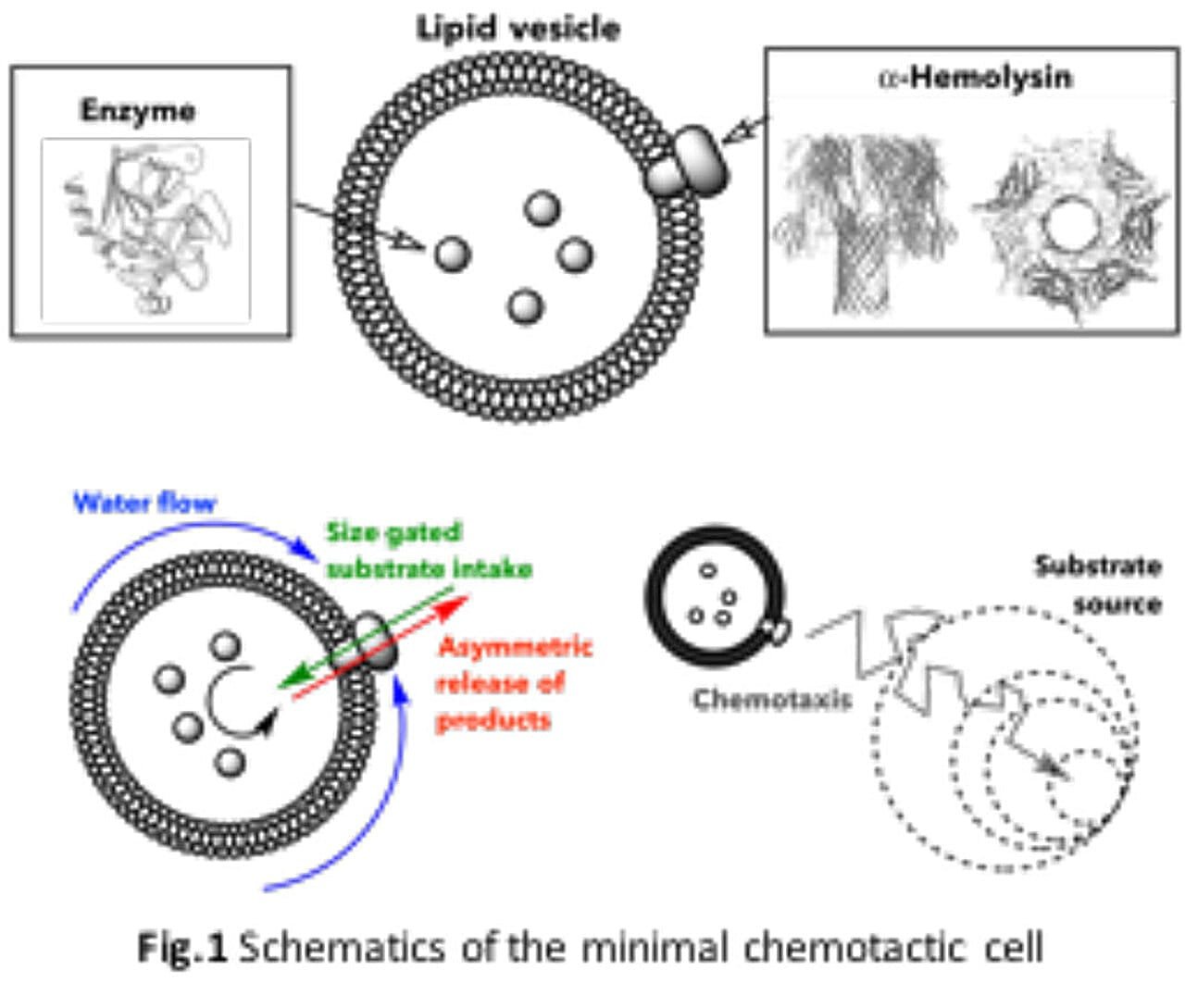
At the heart of this research lies a humble structure: the liposome, a spherical vesicle made from lipids—essentially, a fatty shell that mimics the outer membrane of a biological cell. Inside this shell, the team placed just one type of enzyme: either glucose oxidase or urease. Then they added a single protein pore to the liposome membrane—a tiny doorway through which molecules could pass.
This, in essence, was the entire artificial cell.
It didn’t have DNA. It didn’t need energy reserves. It wasn’t alive in the traditional sense. And yet, when placed in a fluid gradient containing glucose or urea, these minimalist bubbles began to move—consistently and directionally—toward the source of the chemical.
“Watch a vesicle move. Really watch it,” says senior author Professor Giuseppe Battaglia, who leads the Molecular Bionics group at IBEC. “That tiny bubble holds secrets: how cells whisper to each other, how they ship life’s cargo. But biology’s machinery is noisy, too many parts! So, we cheat. We rebuild the whole dance with just three things: a fatty shell, one enzyme, and a pore.”
The beauty lies in its restraint. By stripping away the complexity of a real cell, the researchers revealed the hidden rules underneath—those basic physical and chemical interactions that guide life’s movements long before consciousness ever arrived.
Engines Without Gears
So how exactly do these synthetic cells achieve motion?
The answer is as elegant as it is fundamental: asymmetry. When enzymes inside the vesicle process the incoming molecules—glucose or urea—they generate byproducts. These byproducts leave the vesicle through the pore, creating a concentration imbalance across the membrane.
This imbalance breaks the symmetry of the surrounding fluid. As molecules exit one side of the vesicle faster than the other, tiny flows of liquid are generated along the surface. These flows nudge the vesicle forward, pulling it toward regions with higher concentrations of the target chemical.
The result? Movement—driven by chemistry, guided by physics, and orchestrated by design.
“In a way, the vesicle behaves like a boat,” Fernandes explains. “The enzyme acts like an engine, and the pore serves as its rudder, steering it toward the destination.”
This mechanism, rooted in self-generated gradients and microfluidic forces, is known as self-diffusiophoresis—a process theorized in physics but rarely demonstrated in such a biologically inspired system.
From Chaos to Control
To test their design, the team analyzed over 10,000 vesicles within specially designed microfluidic channels—tiny environments that allowed precise gradients of glucose or urea to be established. They tracked the vesicles’ movements, comparing those with different numbers of pores against those without.
Control vesicles, lacking pores, drifted passively, usually moving toward lower concentrations due to subtle external effects. But the vesicles outfitted with enzyme-pore systems displayed a remarkable reversal: they actively moved up the gradient, toward the source of the substrate.
The more pores added, the stronger the effect. The direction of movement flipped completely. The data were clear. This was not passive diffusion. This was active, targeted migration—chemotaxis.
“It’s remarkable that such a simple system can exhibit such purposeful behavior,” Fernandes says. “It suggests that even the most basic forms of life-like movement don’t necessarily require complexity. They require clever use of fundamental principles.”
The Blueprint of Life, Simplified
What makes this work especially exciting is how biologically relevant it is. Glucose and urea are not exotic compounds—they are everywhere in life. So are enzymes like glucose oxidase and urease. And lipid membranes? They’re the stuff of all cells. The synthetic vesicles mimic not only the structure of biological cells but also their environmental responses—achieving this with materials and mechanisms that nature itself uses.
“These synthetic cells are like blueprints for nature’s navigation system,” says Battaglia. “Build simple, understand profoundly.”
And that’s the deeper ambition behind this work: not just to mimic life, but to reveal it. To study how cells make decisions, how movement emerges from molecular chaos, and how complexity can be reverse-engineered into clarity.
A Synthetic Future with Biological Roots
The implications of this research stretch far beyond academic curiosity. Artificial cells capable of controlled movement could one day be used in targeted drug delivery, navigating the bloodstream to seek out tumors or inflamed tissues. They could be used in environmental cleanup, homing in on pollutants and neutralizing them. Or they could serve as tools in biomedical diagnostics, offering real-time sensing and reporting inside the human body.
But perhaps the most profound use is philosophical: understanding the origins of life itself.
The transition from chemistry to biology—from inert matter to living systems—remains one of science’s greatest mysteries. How did life begin to move, sense, and respond? The artificial cells developed by IBEC offer a glimpse into this transformation.
“We’re trying to understand the minimal requirements for life-like behavior,” Battaglia says. “And in doing so, we may learn how the first cells moved, evolved, and began the journey toward complexity.”
Collaborating Across Disciplines, Across Borders
This study was made possible through a unique collaboration of physicists, biologists, and chemists. Theoretical models by José Miguel Rubí’s team at the University of Barcelona predicted how self-generated gradients could lead to motion. Experimental support came from researchers at University College London, the University of Liverpool, the Biofisika Institute (CSIC-UPV/EHU), and the Ikerbasque Foundation for Science.
It is a vivid example of how interdisciplinary science can solve deeply interdisciplinary problems: the nature of movement, the architecture of cells, the origin of function.
Moving Toward Understanding
In the end, these vesicles—these tiny, shimmering bubbles—are more than laboratory curiosities. They are questions, asked in the language of molecules. How little does it take to imitate life? How simple can complexity become? And what secrets might we uncover when we rebuild biology from the bottom up?
As synthetic biology evolves, the ability to design systems that behave like life—without necessarily being alive—challenges our definitions and deepens our understanding. With every step these artificial cells take, we move closer to deciphering the quiet genius of evolution and the graceful mechanics that animate our world.
In a whisper of fluid, in the twitch of a vesicle, the story of life begins again—from the simplest parts, beautifully reassembled.
Reference: Barbara Borges Fernandes et al, The minimal chemotactic cell, Science Advances (2025). DOI: 10.1126/sciadv.adx9364. www.science.org/doi/10.1126/sciadv.adx9364