Inside every cell of your body, from the neurons firing in your brain to the muscle fibers contracting in your heart, lies a collection of tiny, bean-shaped organelles with an ancient and extraordinary story. These structures are known as mitochondria. They are so small that millions can fit in a single drop of blood, and yet they wield enormous influence over the life and energy of every living being.
To understand life at its most fundamental level, we must peer not just into the structure of cells, but into the engines that fuel them. Mitochondria are often described as the “powerhouse of the cell”—a phrase so overused in textbooks that it risks becoming a cliché. But this title is not hyperbole. Mitochondria are responsible for producing most of the energy a cell needs to survive, grow, divide, and communicate. Without them, multicellular life—plants, animals, humans—would not be possible.
They are not just power producers, though. Mitochondria are storytellers of evolution, regulators of cell death, guardians of cellular health, and even co-authors of our genetic code. Their influence extends from metabolism and immunity to aging and disease. Understanding mitochondria is not merely an academic exercise. It’s an exploration of the machinery of life itself.
The Evolutionary Odyssey of Mitochondria
To fully appreciate mitochondria, we need to rewind over 1.5 billion years into the depths of evolutionary time. Long before animals or plants, the Earth was inhabited by simple single-celled organisms—bacteria and archaea. Somewhere along this ancient microbial landscape, an extraordinary event occurred: a large primitive host cell engulfed a smaller, energy-efficient bacterium. But instead of digesting it, the two formed an alliance.
This symbiotic relationship was not a random accident but a moment of evolutionary genius. The engulfed bacterium, capable of converting oxygen and nutrients into energy with remarkable efficiency, became a permanent resident inside the host cell. Over millennia, it lost much of its independence, surrendering many of its genes to the host’s nucleus. But it retained enough autonomy to continue producing energy. Thus, mitochondria were born—from bacteria that had once lived free.
This theory, known as endosymbiosis, was championed by the biologist Lynn Margulis in the 20th century. Though initially met with skepticism, it is now widely accepted and supported by genetic evidence. Mitochondria, in fact, still carry their own DNA—a ghostly relic of their ancient bacterial past.
That bacterial ancestry is not merely a curiosity. It explains why mitochondria resemble bacteria in shape and structure, why they divide independently of the cell, and why they are so central to life on Earth. The energy boost from mitochondria allowed cells to grow larger and more complex, paving the way for the evolution of multicellular organisms. From this tiny merger came the spark that ignited the diversity of life.
Anatomy of an Energy Factory
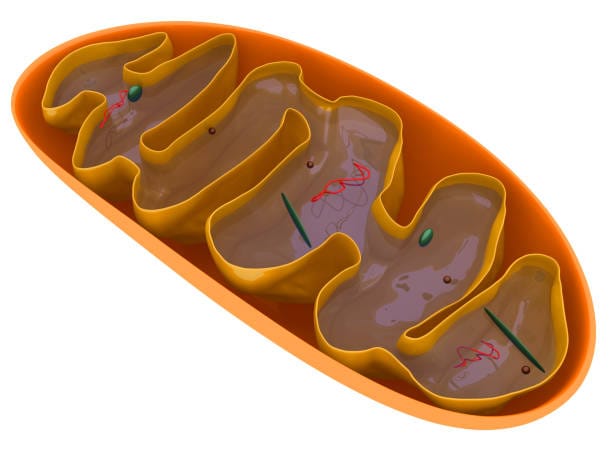
To understand how mitochondria generate energy, we must step inside their double membrane—a unique feature that reflects their bacterial origins. The outer membrane is smooth, but the inner membrane is intricately folded into structures called cristae, which vastly increase surface area. This inner membrane houses the protein complexes responsible for a process known as oxidative phosphorylation.
Energy production begins with nutrients—glucose, fatty acids, and amino acids—entering the mitochondria. These fuels undergo a series of reactions collectively called the citric acid cycle, or Krebs cycle. As these molecules are broken down, they release high-energy electrons. These electrons are shuttled through the electron transport chain, a series of protein complexes embedded in the inner membrane.
As electrons flow through this chain, they create a proton gradient across the membrane—much like water building up behind a dam. This gradient powers the ATP synthase enzyme, which spins like a turbine to produce adenosine triphosphate (ATP), the universal energy currency of cells.
ATP is not just a molecule—it’s life’s coin. It powers everything from muscle contractions and nerve impulses to protein synthesis and DNA replication. A single human cell can contain thousands of mitochondria, churning out millions of ATP molecules every second. It is a feat of engineering so elegant, so finely tuned, that it has no human-made equal.
Beyond Energy: A Multifaceted Organelle
Although best known for producing ATP, mitochondria play a vast and diverse set of roles in cellular biology. They are not mere energy factories—they are decision-makers, quality control agents, and even executioners.
One of their lesser-known roles involves apoptosis, or programmed cell death. When a cell becomes damaged beyond repair or infected by a virus, mitochondria can release proteins that trigger a cascade of events leading to the cell’s orderly self-destruction. Far from being a flaw, this ability is essential for health—it prevents the proliferation of damaged cells and forms the sculpting mechanism in embryonic development.
Mitochondria also regulate calcium homeostasis, buffer oxidative stress, and synthesize key molecules like steroids and heme groups (which are essential for hemoglobin). They are critical participants in immune signaling, aging, and inflammation. When mitochondria malfunction, the consequences are far-reaching—affecting virtually every organ system.
What makes mitochondria so remarkable is their responsiveness. They adapt to the cell’s energy demands, multiply when needed, fuse to share resources, and break apart when damaged. This constant remodeling, called mitochondrial dynamics, ensures that each mitochondrion is optimized for survival.
Mitochondrial DNA: A Genetic Time Capsule
Inside each mitochondrion resides a small, circular genome, separate from the DNA found in the nucleus. Human mitochondrial DNA (mtDNA) contains just 37 genes, but these genes are absolutely essential. They encode components of the electron transport chain as well as the machinery to translate those genes into proteins.
The presence of this DNA is more than a molecular quirk—it’s a key to understanding ancestry, disease, and evolution. Mitochondrial DNA is inherited maternally, meaning it passes from mother to child with few mutations. This feature has allowed scientists to trace human migration patterns over tens of thousands of years. By studying mtDNA, researchers identified “Mitochondrial Eve,” a hypothetical woman who lived in Africa around 150,000 to 200,000 years ago and from whom all modern humans descend maternally.
But mitochondrial DNA is also vulnerable. Unlike nuclear DNA, it has limited repair mechanisms and is exposed to the reactive byproducts of energy production—free radicals. These factors make it prone to mutations, some of which can cause mitochondrial diseases.
When Power Fails: Mitochondrial Diseases and Dysfunction
Because mitochondria are involved in so many vital functions, their dysfunction can lead to a wide range of diseases. Mitochondrial diseases are a group of disorders caused by mutations in mitochondrial DNA or in nuclear genes that affect mitochondrial function. These conditions can be devastating, affecting the brain, heart, muscles, and other high-energy tissues.
Symptoms vary widely but often include muscle weakness, neurological problems, heart disease, diabetes, and fatigue. These diseases are often progressive and difficult to treat. In recent years, researchers have begun exploring therapies like gene editing, mitochondrial replacement therapy, and dietary interventions to manage symptoms and slow progression.
But mitochondrial dysfunction is not limited to rare genetic diseases. It is increasingly recognized as a contributor to common conditions such as Alzheimer’s disease, Parkinson’s disease, cancer, metabolic syndrome, and aging. In each of these cases, impaired mitochondrial function leads to increased oxidative stress, inflammation, and cellular damage.
Understanding how and why mitochondria fail is one of the most pressing challenges in modern medicine—and may hold the key to extending healthspan and treating chronic disease.
Mitochondria and Aging: The Quiet Erosion of Power
Why do we age? The question has haunted humanity for millennia, and while no single answer has emerged, mitochondria are at the heart of many theories.
One widely accepted idea is the mitochondrial free radical theory of aging. It posits that as mitochondria produce energy, they also generate reactive oxygen species (ROS)—unstable molecules that can damage DNA, proteins, and lipids. Over time, this oxidative stress accumulates, impairing mitochondrial function and leading to cellular decline.
Recent research has nuanced this theory. It suggests that a moderate level of ROS may actually signal beneficial adaptations—a phenomenon known as mitohormesis. But when ROS levels become too high, or when mitochondrial DNA mutations accumulate beyond a threshold, cells may enter a state of senescence or apoptosis.
The link between mitochondria and aging is not just theoretical. In animal models, enhancing mitochondrial function extends lifespan. In humans, lifestyle factors that support mitochondrial health—exercise, fasting, sleep, and proper nutrition—are associated with greater vitality and longevity. The mitochondrion may not be the only reason we age, but it is certainly one of the central players.
The Mitochondrial Renaissance: Cutting-Edge Research and Hope for the Future
In recent years, mitochondria have experienced a scientific renaissance. Once relegated to the background of cell biology, they are now front and center in research on aging, metabolism, neurodegeneration, and personalized medicine.
One of the most exciting developments is mitochondrial replacement therapy (MRT), a technique that allows women with faulty mitochondria to have healthy children. In MRT, the nuclear DNA from a mother’s egg is transferred to a donor egg with healthy mitochondria, resulting in a child with three genetic contributors. While controversial, this approach has already led to the birth of several healthy children and may prevent the transmission of debilitating mitochondrial diseases.
Gene-editing technologies like CRISPR are also being adapted to target mitochondrial DNA mutations. Though editing mtDNA is more challenging than nuclear DNA, researchers are making progress with tools like mitochondrially targeted base editors.
Meanwhile, scientists are exploring compounds that boost mitochondrial biogenesis (the creation of new mitochondria), enhance their dynamics, or improve their efficiency. From NAD+ precursors to senolytics and mitochondrial-targeted antioxidants, a new class of therapies is emerging with the potential to rejuvenate cells and combat age-related diseases.
Living for Your Mitochondria: Lifestyle and Cellular Energy
The way you live affects your mitochondria profoundly. Physical exercise, especially aerobic and high-intensity interval training, increases mitochondrial density and function. Fasting and caloric restriction activate pathways that promote mitochondrial health, including sirtuins and AMPK. Sleep is when cells repair mitochondrial damage. Even exposure to sunlight and cold can stimulate mitochondrial resilience.
Conversely, chronic stress, poor diet, sedentary behavior, and toxins can impair mitochondrial function. Environmental pollutants, heavy metals, processed foods, and excess sugar create oxidative stress that overwhelms mitochondria and accelerates aging.
In a sense, every decision you make is a dialogue with your mitochondria. They respond to the cues of your environment, the rhythms of your lifestyle, and the nutrients in your food. And in turn, they shape your energy, your resilience, and your health.
The Poetry of Power: A Final Reflection
To study mitochondria is to witness the poetry of biology. These ancient organelles are not just machines churning out ATP—they are the echoes of life’s deep past, sculptors of our present vitality, and architects of our future health. They remind us that life is not just about surviving but thriving. That energy is not simply a chemical reaction but the spark that animates every breath, every heartbeat, every thought.
The phrase “powerhouse of the cell” doesn’t quite do justice to the richness of their role. Mitochondria are time travelers from an ancient world, biochemists that convert fuel into life, and sentinels that decide when a cell should live or die. They are at once the foundation and the frontier of biology.
So the next time you feel your heart racing, your muscles burning, or your thoughts soaring—thank your mitochondria. Inside you, in every cell, are the flickers of a billion-year-old alliance that made you possible.