Within every living cell, tiny, invisible machines work with relentless precision. These machines don’t have gears, wires, or motors. They are made of RNA and proteins, and yet they perform one of the most essential functions in biology: building the proteins that make life possible. These cellular architects are called ribosomes. Small enough to go unnoticed by even the most powerful light microscopes, ribosomes are nonetheless vital to the survival of every organism—from the smallest bacterium to the most complex human brain.
To understand life, you must understand proteins. And to understand proteins, you must understand ribosomes. These structures are not merely passive participants in cell biology. They are dynamic, complex, and fascinating molecular machines whose precision and efficiency have evolved over billions of years. They are both the product and the creators of the biological world, translating genetic information into action and identity. In this article, we will explore the story of ribosomes—their intricate structure, their fundamental role in protein synthesis, and the awe-inspiring choreography they perform in every living cell.
The Discovery That Changed Everything
The existence of ribosomes was suspected before they were seen. In the early 20th century, scientists began piecing together the mystery of how cells make proteins. The discovery of DNA and RNA in the 1940s and 1950s hinted at a system of information transfer—but the missing link between the code and the product remained elusive.
It wasn’t until the development of the electron microscope that ribosomes were first observed. In the 1950s, George Palade used electron microscopy to identify small granular structures in the cytoplasm of animal cells. These granules, later named ribosomes, were seen studding the surface of the endoplasmic reticulum and floating freely in the cytosol. Palade’s work earned him the Nobel Prize in 1974, and ribosomes quickly took center stage in molecular biology.
The more scientists studied ribosomes, the more complex and beautiful they appeared. These were not simple blobs of molecules. They were intricate machines, composed of both RNA and proteins, capable of decoding genetic information and assembling amino acids with extraordinary accuracy and speed.
Architecture of a Molecular Machine
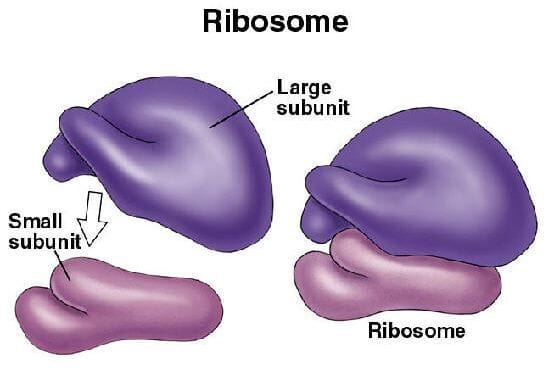
At first glance, a ribosome may seem like a jumbled clump of molecules. But look closer, and its architecture reveals a structure both elegant and efficient. Ribosomes are composed of two major subunits: a large subunit and a small subunit. These subunits are made from ribosomal RNA (rRNA) and a variety of ribosomal proteins.
The sizes of the subunits are typically described by their sedimentation coefficients, measured in Svedberg units (S). In prokaryotic cells like bacteria, the small subunit is 30S, and the large subunit is 50S, combining to form a functional 70S ribosome. In eukaryotic cells, the small and large subunits are 40S and 60S respectively, forming the larger 80S ribosome.
What makes these numbers fascinating is that they don’t add up arithmetically—their values reflect not mass but the rate at which they sediment during centrifugation, which depends on shape and density. This detail alone hints at the intricate physical nature of ribosomes. They’re not just linear accumulations of molecules; they are spatially organized and folded in ways that maximize their functional power.
Within each subunit lies a carefully arranged matrix of rRNA, which serves not just as a scaffold, but as a chemically active component. In fact, it is the rRNA—not the proteins—that carries out the critical steps of peptide bond formation. This was a revolutionary insight: the ribosome is not a protein enzyme but a ribozyme, an RNA-based catalyst. The implications are profound, suggesting that RNA was central to the earliest stages of life—a hypothesis known as the RNA world theory.
Translation: Turning Genes Into Function
At the heart of the ribosome’s function is translation: the process of reading messenger RNA (mRNA) and using its information to build proteins. This process is one of the central dogmas of molecular biology: DNA is transcribed into mRNA, which is then translated into protein. Ribosomes are the interpreters of this genetic code.
Translation begins when the small ribosomal subunit binds to the mRNA strand. It scans the strand until it finds a start codon—usually AUG, which codes for the amino acid methionine. This codon signals the beginning of a protein-coding sequence. Once the start codon is recognized, the large subunit joins the small one, forming a complete ribosome. From this point forward, the ribosome proceeds along the mRNA, three nucleotides at a time, reading each codon and recruiting the corresponding amino acid.
A critical player in this process is transfer RNA (tRNA), which acts as a molecular courier. Each tRNA molecule carries a specific amino acid and has an anticodon that matches the mRNA codon. When the ribosome encounters a codon, a tRNA with the complementary anticodon enters the ribosome, bringing along its amino acid. The ribosome then forms a peptide bond between this amino acid and the growing protein chain.
The ribosome has three binding sites for tRNA: the A (aminoacyl) site, the P (peptidyl) site, and the E (exit) site. This triad of functional zones allows the ribosome to coordinate the orderly addition of amino acids, moving the tRNAs through a precisely choreographed sequence of events. With each cycle, the protein grows longer, folding into its functional shape even as it emerges from the ribosome.
Precision and Speed: An Unmatched Efficiency
The ribosome is an astonishing example of biological efficiency. It can translate a protein at a rate of 10 to 20 amino acids per second in eukaryotic cells—and even faster in bacteria. Given that some proteins consist of thousands of amino acids, this is no small feat.
But it’s not just about speed; it’s about accuracy. The ribosome makes fewer than one mistake in 10,000 amino acid additions—a level of precision far better than many man-made machines. It achieves this by a combination of proofreading mechanisms, molecular recognition, and kinetic controls. The tRNA must fit perfectly into the ribosome’s active site. If the match is incorrect, the tRNA is rejected, and translation pauses until the correct tRNA is found.
The elegance of this system becomes even more apparent when one considers that all of this happens without conscious direction. The ribosome is not “aware” of what it’s doing—it simply follows the chemical rules inscribed in its structure and honed by evolution. Yet, the result is a process that produces every enzyme, hormone, receptor, and structural protein in your body. In a very real sense, ribosomes are what make you… you.
Free vs. Bound: The Ribosomal Highway System
Ribosomes come in two main varieties: free and membrane-bound. Free ribosomes float in the cytosol and produce proteins that function in the cytoplasm or in organelles like the nucleus and mitochondria. Bound ribosomes are attached to the rough endoplasmic reticulum (RER), giving it its characteristic studded appearance under a microscope.
The destination of a protein often depends on where it’s synthesized. If the protein is meant to be secreted from the cell, embedded in the membrane, or delivered to lysosomes, its translation usually begins on a free ribosome. But as soon as a signal peptide is synthesized—a short amino acid sequence that acts like a postal code—the ribosome docks onto the RER and continues translation into the lumen of the ER.
This division of labor allows the cell to maintain organization and ensure that proteins reach their correct destinations. It also underscores the remarkable adaptability of ribosomes, which can shift between free-floating and membrane-bound states depending on cellular needs.
Ribosomes in Prokaryotes and Eukaryotes: A Shared Heritage
Though ribosomes are universal, there are important differences between prokaryotic and eukaryotic versions. Prokaryotic ribosomes are smaller and simpler, with fewer proteins and shorter rRNA chains. Eukaryotic ribosomes are larger and more complex, with more layers of regulation and more sophisticated control mechanisms.
Despite these differences, the core function remains the same. The similarities in structure and function across all domains of life strongly support the idea of a common evolutionary origin. In fact, ribosomal RNA sequences are so conserved that they are often used to determine evolutionary relationships among organisms. This molecular fingerprinting has revolutionized taxonomy, leading to the reclassification of entire groups of organisms based on ribosomal genetics.
Even the mitochondria and chloroplasts within eukaryotic cells contain their own ribosomes, which resemble those of bacteria. This supports the endosymbiotic theory, which posits that these organelles originated from once-independent prokaryotic cells that were engulfed by ancestral eukaryotes. Their ribosomes are a molecular time capsule, offering a glimpse into the deep past of cellular evolution.
Antibiotics and the Ribosomal Battleground
One of the most practical reasons for studying ribosomes comes from medicine. Many antibiotics target bacterial ribosomes, exploiting the differences between bacterial and human ribosomes to inhibit protein synthesis in pathogens without harming host cells.
Drugs like tetracycline, erythromycin, and streptomycin bind to specific sites on bacterial ribosomes and disrupt their function. This halts protein production, crippling the bacteria and giving the immune system a chance to eliminate the infection.
However, this microbial battleground is also a site of evolutionary arms races. Bacteria can develop resistance by mutating their ribosomal RNA or producing enzymes that inactivate antibiotics. Understanding ribosomal structure in even greater detail is essential for developing the next generation of antimicrobial therapies.
Ribosomes and the Origins of Life
Beyond their medical and cellular importance, ribosomes are central to some of the deepest questions in science: How did life begin? What was the first molecule capable of replication and function?
Many scientists believe that ribosomes hold a clue. The fact that the catalytic core of the ribosome is RNA—not protein—suggests that RNA once played a dual role as both genetic material and functional catalyst. In the hypothesized RNA world, early life forms may have relied on RNA to store information and perform chemical reactions. Ribosomes, with their RNA-based catalytic activity, may be evolutionary relics from this era.
This idea is not mere speculation. Structural studies have shown that the most ancient parts of the ribosome—the peptidyl transferase center and the decoding site—are composed almost entirely of RNA, with proteins only later additions to stabilize and regulate the structure. It’s as if proteins joined the party after RNA had already built the house.
Understanding the ribosome is thus not only a window into the present—it’s a portal to the very origins of biology itself.
Beyond the Textbooks: A Living Symphony
It’s tempting to view ribosomes as static textbook diagrams: colored blobs labeled with letters and arrows. But in reality, they are living, writhing symphonies of movement. Cryo-electron microscopy and high-speed imaging have revealed the ribosome in action, showing how it swivels, bends, and breathes as it builds proteins.
These images are not just visually stunning—they are emotionally powerful. They remind us that even in the tiniest corners of life, there is drama, complexity, and beauty. The ribosome is not merely a translator of code—it is a storyteller, shaping the molecules that make memory, motion, thought, and life itself.
The Future of Ribosome Research
Despite decades of study, the ribosome continues to yield surprises. Scientists are now exploring how ribosomes vary subtly across tissues, organisms, and even developmental stages. The concept of “specialized ribosomes”—ribosomes with slightly different compositions tailored to specific cellular needs—is a hot area of research.
Biotechnologists are also engineering ribosomes to create novel proteins or incorporate synthetic amino acids—steps toward expanding the genetic code and developing entirely new classes of biomolecules. These efforts could revolutionize medicine, agriculture, and materials science.
Moreover, understanding ribosome malfunctions is crucial for decoding diseases like cancer, neurodegeneration, and rare genetic disorders known as ribosomopathies. These conditions, rooted in errors of translation, show just how critical ribosomes are to health and development.
The Molecular Bridge Between Information and Identity
The ribosome is a marvel of biology—a bridge between the abstract world of genetic information and the tangible world of proteins and functions. It is where identity is forged, where code becomes flesh.
Every heartbeat, every breath, every thought relies on proteins that were born in ribosomes. And every ribosome operates not with consciousness, but with a kind of molecular wisdom—a precision and elegance sculpted by four billion years of evolution.
In the end, to understand ribosomes is to glimpse the engine room of life itself. They are not merely parts of cells. They are the artisans of existence, the unsung heroes in the story of what it means to be alive.