Imagine shrinking yourself down to a size smaller than a dust mite, smaller than a bacterium, until you’re inside a single living cell. It’s not empty or still—it’s teeming with bustling activity, complex architecture, and microscopic machinery working in perfect harmony. Among the intricate corridors and chambers, you would encounter a labyrinthine structure sprawling through the cell like a biological superhighway. This is the endoplasmic reticulum (ER), a marvel of cellular engineering and a central hub for life’s most vital processes.
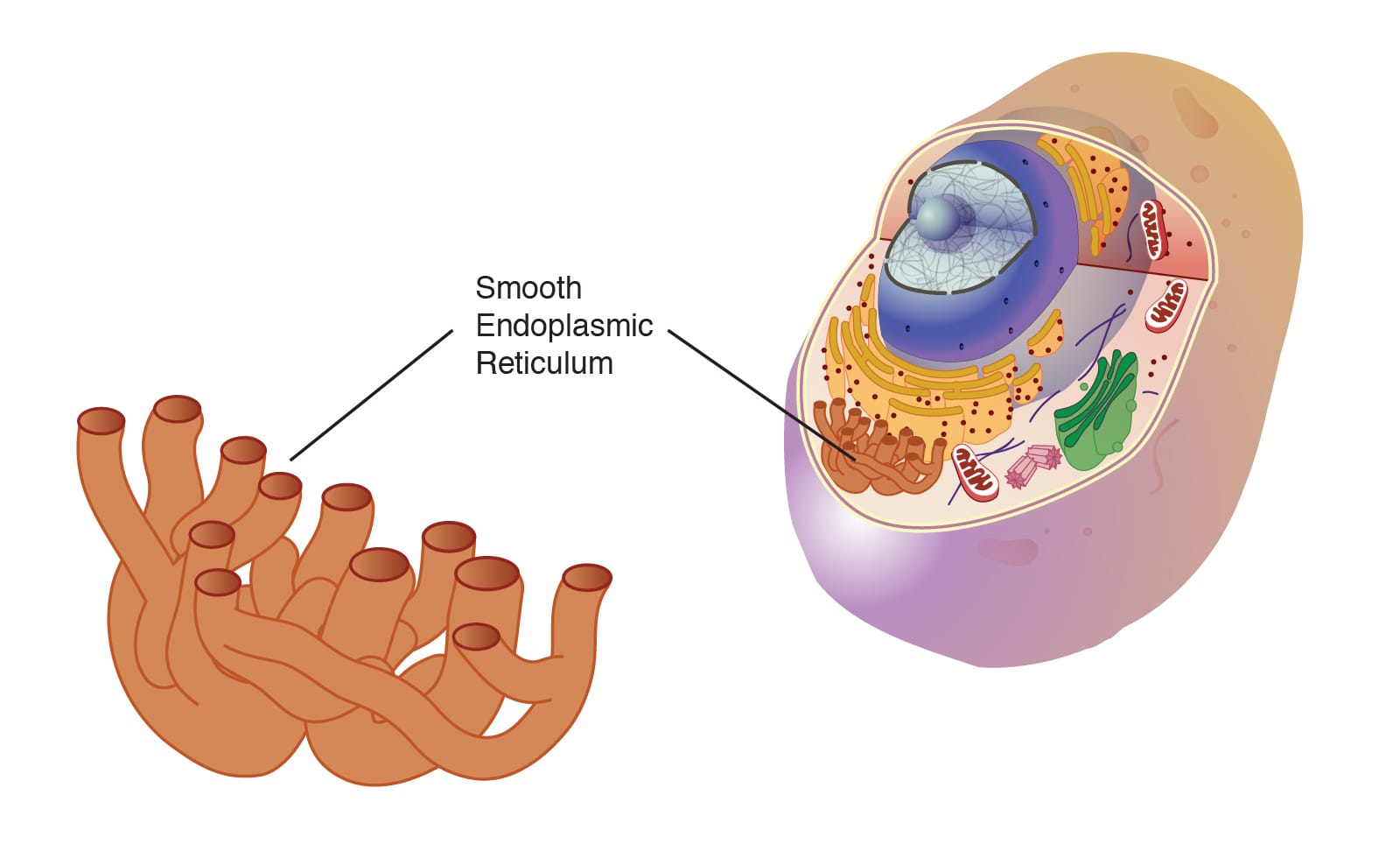
The endoplasmic reticulum isn’t as famous as DNA or mitochondria, yet it is every bit as essential. It’s a multitasking organelle that helps build proteins, produce lipids, store calcium, and detoxify harmful substances. At first glance, it might seem like one structure—but in truth, it comes in two distinct forms: the rough endoplasmic reticulum (RER) and the smooth endoplasmic reticulum (SER). Each performs specialized, yet deeply interconnected, roles that keep cells alive, adapting, and thriving.
To understand the difference between rough and smooth ER, we must first explore the greater story of cell biology—the language of life written not in words, but in membranes, molecules, and microscopic factories.
The Architecture of Cellular Infrastructure
The endoplasmic reticulum is a continuous membrane system that stretches throughout the cytoplasm of eukaryotic cells. This membrane is connected directly to the outer layer of the nuclear envelope—the double membrane that surrounds the cell’s nucleus, where DNA is stored and transcribed into RNA. This intimate connection between nucleus and ER reflects their functional relationship: the nucleus issues the genetic instructions, and the ER helps execute them.
The ER itself is composed of flattened sacs and branching tubules called cisternae, and it’s within this network that two distinct forms emerge: the rough ER and the smooth ER. While they share the same membrane system and often transition seamlessly into each other, they look and function very differently.
The rough ER appears studded with tiny dots under a microscope—ribosomes, the molecular machines that build proteins. This gives it a “rough” texture and clues us in to its primary function: protein synthesis. In contrast, the smooth ER lacks ribosomes, giving it a glossy, tubular appearance. It is the domain of lipid production, detoxification, and calcium storage.
Both types of ER are dynamic and adaptable. Depending on a cell’s function—whether it’s a neuron, liver cell, muscle fiber, or hormone-secreting gland—the balance between rough and smooth ER shifts. Each cell tailors its ER to meet its unique demands, revealing just how versatile and vital this organelle truly is.
The Rough Endoplasmic Reticulum: Assembly Line of Life
The rough endoplasmic reticulum is the cell’s protein factory. Its surface is covered in ribosomes—tiny, powerful complexes made of RNA and protein—that translate messenger RNA (mRNA) into polypeptide chains. These chains fold into functional proteins, which will go on to serve as enzymes, structural components, receptors, or signaling molecules.
But not all protein-making ribosomes are attached to the ER. Some float freely in the cytoplasm. So why anchor them to the ER at all? The key lies in the destination of the proteins being made. Ribosomes on the rough ER synthesize proteins that are destined to be:
- Secreted from the cell (like hormones or antibodies)
- Incorporated into the cell membrane (like ion channels and receptors)
- Sent to other organelles, especially the lysosomes and Golgi apparatus
As the ribosome translates an mRNA strand, it feeds the growing polypeptide into the ER lumen—the internal space enclosed by the ER membrane. Inside, molecular chaperones assist in folding the protein properly, while enzymes attach chemical groups like carbohydrates to create glycoproteins. This post-translational modification is crucial for the protein’s stability and function.
The rough ER doesn’t work in isolation. Once a protein is synthesized and modified, it’s packaged into transport vesicles—tiny membrane-bound bubbles—and sent to the Golgi apparatus for further processing and sorting. This journey is part of the secretory pathway, a tightly choreographed system that ensures proteins reach the right destination at the right time.
Some cells, like pancreatic acinar cells or plasma B-cells of the immune system, are packed with rough ER. These cells specialize in churning out digestive enzymes or antibodies, and they rely on the rough ER to meet the enormous demand.
The rough ER is more than just a production line. It’s a quality control center. Proteins that fail to fold correctly are retained and degraded, preventing potential harm. In fact, when too many misfolded proteins accumulate, the cell activates the unfolded protein response (UPR)—a stress signaling system that can slow down translation, increase chaperone production, or even trigger cell death if the damage is irreparable.
In this way, the rough ER acts as both a workshop and a watchdog, ensuring that only properly crafted proteins make their way into the world.
The Smooth Endoplasmic Reticulum: Silent Chemist of the Cell
If the rough ER is the cell’s assembly line, the smooth ER is its chemical laboratory. Lacking ribosomes, the smooth ER focuses on a different set of tasks—tasks no less vital than protein synthesis.
One of its most important functions is lipid metabolism. Within the smooth ER membrane, enzymes catalyze the synthesis of phospholipids and cholesterol—crucial components of cellular membranes. These lipids maintain the fluidity, flexibility, and integrity of the membranes that enclose the cell and its organelles.
Beyond lipids for membranes, the smooth ER also produces steroid hormones in endocrine cells. In the adrenal glands, testes, and ovaries, the smooth ER generates cortisol, testosterone, estrogen, and other hormones from cholesterol. These powerful molecules regulate stress, metabolism, reproduction, and more—tiny messengers with massive influence.
The smooth ER also plays a starring role in detoxification, particularly in liver cells. Enzymes embedded in the ER membrane—especially members of the cytochrome P450 family—help neutralize drugs, alcohol, and harmful metabolic byproducts. They do this by oxidizing, reducing, or hydrolyzing toxins, making them easier to excrete. This function has enormous medical relevance, influencing how drugs are metabolized and how quickly they leave the body.
Another vital function of the smooth ER is calcium storage. In muscle cells, a specialized form of smooth ER called the sarcoplasmic reticulum controls calcium ion release, which triggers muscle contraction. Elsewhere, calcium release from the ER can initiate signaling cascades, control gene expression, and even determine whether a cell lives or dies.
The smooth ER is dynamic. It can expand or shrink depending on a cell’s needs. For instance, when a liver cell is exposed to toxins, it increases its smooth ER content to ramp up detoxification. When hormone production is in high demand, steroid-producing cells enlarge their smooth ER to meet the challenge.
While it works quietly behind the scenes, the smooth ER is a master chemist, handling the fluid molecules of life with exquisite control.
Collaboration, Not Competition
Although rough and smooth ER have distinct functions, they are not rivals but partners. Their membranes are continuous, and their work often intersects. For example, newly synthesized proteins in the rough ER may embed themselves in the membrane, influencing the activities of the smooth ER’s detoxification enzymes. Likewise, lipids synthesized in the smooth ER may be used to expand the rough ER membrane or assemble vesicles for protein transport.
The ER also works closely with other organelles. It hands off proteins to the Golgi apparatus for modification and distribution. It exchanges lipids with mitochondria to maintain membrane health. It communicates with endosomes and peroxisomes. It even forms contact sites with the plasma membrane to regulate calcium flow and lipid transfer.
This organelle, often overlooked in textbooks, is in truth a central coordinator of cellular life—a networked, multifunctional hub that keeps the cell running smoothly.
When the ER Fails: Disease and Dysfunction
Because the endoplasmic reticulum plays such a central role in so many critical processes, its malfunction can be catastrophic. ER stress and dysfunction are implicated in a growing list of diseases, from neurodegeneration to diabetes to cancer.
In Alzheimer’s disease, for instance, the accumulation of misfolded amyloid-beta proteins overwhelms the ER’s quality control system, leading to chronic stress and cell death. In type 2 diabetes, ER stress in insulin-producing beta cells contributes to their dysfunction and eventual loss. In some cancers, the ER is hijacked to produce large quantities of proteins that help tumors grow and evade the immune system.
Viruses also exploit the ER. The coronavirus responsible for COVID-19, for example, manipulates the ER to create viral replication compartments—membrane-bound bubbles where new viral genomes are assembled and protected from immune detection.
These insights have spurred intense interest in the ER as a therapeutic target. Researchers are investigating ways to modulate the unfolded protein response, enhance ER stress resistance, or block viral hijacking. The hope is that by better understanding and controlling ER function, we can develop new treatments for some of the most challenging diseases of our time.
Evolutionary Origins and Future Perspectives
The endoplasmic reticulum is not unique to humans or animals—it is found in all eukaryotic cells, from yeast to plants to protists. Its origin dates back more than a billion years, likely evolving from invaginations of the plasma membrane in ancient proto-eukaryotic cells. As complexity grew, so did the ER, diversifying into rough and smooth forms to meet the demands of more specialized cellular life.
This evolutionary perspective helps us appreciate the ER not as a static structure, but as a dynamic invention—a flexible system that adapts and transforms with the needs of the organism.
In modern biotechnology, synthetic biologists are now looking to harness and reengineer the ER’s capabilities. Can we design cells that manufacture custom proteins with enhanced efficiency? Can we rewire ER pathways to detoxify pollutants or produce valuable pharmaceuticals? The answers lie in deepening our understanding of how this remarkable structure works.
A Microscopic Symphony
The endoplasmic reticulum is, in many ways, the unsung hero of cellular life. While DNA provides the blueprint and mitochondria generate the energy, the ER carries out the construction, refinement, and delivery of life’s most essential materials. It balances complexity and order, folding the proteins that define who we are and building the lipids that shield and structure us.
To see the ER in action is to witness a microscopic symphony—a carefully orchestrated performance where every ribosome, enzyme, vesicle, and ion plays its part. It’s a testament to nature’s ingenuity, hidden in every heartbeat, every breath, and every thought we have.
So the next time you read a label on a pill bottle, marvel at the precision of muscle movement, or learn about a breakthrough in medicine, remember: somewhere deep in your cells, the rough and smooth endoplasmic reticulum are working tirelessly behind the scenes. Shaping life. Maintaining balance. And reminding us that even the most powerful forces can begin with something unseen.