In the stillness of a microscope slide lies a world more intricate than any city, more coordinated than any orchestra. Within each living cell, countless processes unfold simultaneously, giving rise to the miracle we call life. And amid this microscopic bustle, nestled among other organelles, stands one of biology’s most elegant and essential structures: the Golgi apparatus.
To the untrained eye, the Golgi apparatus is a collection of flattened sacs, slightly curved like a stack of pancakes. But beneath its modest appearance lies a sophisticated processing center—one that determines the fate of proteins, shapes the identity of cells, and ensures that the internal machinery of life runs smoothly. If the cell were a postal system, the Golgi would be its central post office—labeling, packaging, and shipping molecules with precision that borders on the miraculous.
Yet, despite its vital role in cellular biology, the Golgi apparatus rarely captures the imagination the way DNA, mitochondria, or the brain do. It’s often overlooked in textbooks and dismissed in popular science. But peel back the membranes, peer into its layered folds, and you’ll find a structure of breathtaking complexity—one that deserves our full attention and admiration.
The Man Behind the Membranes
The story of the Golgi apparatus begins with an Italian scientist named Camillo Golgi, whose keen eye and pioneering spirit forever changed our understanding of cellular structure. In the late 19th century, cell biology was still in its infancy. The inner workings of cells remained largely mysterious, and staining techniques were rudimentary. But Golgi was determined to see deeper.
In 1898, using a silver nitrate staining method he had developed himself—dubbed the “black reaction”—Golgi observed a network of thread-like structures in nerve cells. He initially referred to the discovery as the “internal reticular apparatus,” a term that reflected its net-like appearance. It was a daring claim at the time, and not everyone believed him. Some scientists thought the structure was an artifact of the staining process.
But as microscopy improved, Golgi’s discovery was validated. The structure was real—and ubiquitous. Nearly every eukaryotic cell possessed it, from single-celled protists to complex multicellular organisms. In honor of his contribution, the structure was renamed the Golgi apparatus, or sometimes simply the Golgi.
Golgi’s legacy extended beyond his eponymous organelle. He shared the 1906 Nobel Prize in Physiology or Medicine with Santiago Ramón y Cajal for their work on the nervous system, though ironically, the two men held opposing views on neural organization. Golgi died in 1926, but his apparatus—his “beautiful network”—continues to shape the life of every cell.
Peering into the Golgi: A Master of Modification
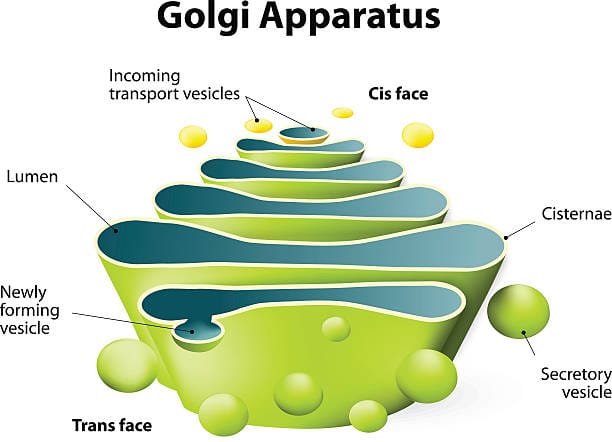
At first glance, the Golgi apparatus resembles a stack of pita bread or a bundle of flattened balloons. These flattened, membrane-bound sacs are called cisternae, and they’re arranged in a precise, polarized stack. One side of the stack faces the endoplasmic reticulum (ER)—this is the cis face. The other side, called the trans face, looks toward the plasma membrane or the cell’s exterior.
But this structural orientation is more than anatomical trivia; it reflects a directional flow of traffic. Proteins and lipids synthesized in the ER are sent to the Golgi’s cis face, where they begin a journey through the stack. As they travel from cis to trans, they undergo a series of carefully orchestrated chemical modifications. These can include the addition of sugar chains (glycosylation), phosphate groups (phosphorylation), sulfate groups (sulfation), and more.
The Golgi is not a passive conduit but an active and discerning editor. It customizes proteins for their final destinations, adding molecular tags that act like shipping labels. These chemical additions are not arbitrary—they determine where the molecule will go next, whether it’s embedded in the cell membrane, secreted into the bloodstream, or transported to another organelle.
For example, a digestive enzyme destined for the lysosome might be tagged with a mannose-6-phosphate marker in the Golgi. Without this label, the enzyme might be shipped to the wrong place—or not shipped at all. Like an airport control tower guiding planes, the Golgi ensures every molecule departs with the correct itinerary and reaches its destination intact.
The Symphony of Secretion
In specialized cells—especially those that secrete hormones, enzymes, or neurotransmitters—the Golgi apparatus plays an especially prominent role. Consider the pancreatic cells that release insulin, or the immune cells that secrete antibodies. Each of these products must be synthesized, modified, sorted, and shipped with impeccable accuracy. The Golgi acts as the conductor of this cellular symphony.
Once modified in the Golgi, proteins are packaged into vesicles—tiny, membrane-bound bubbles that bud off from the trans face of the Golgi stack. These vesicles contain the necessary instructions encoded in their molecular tags, guiding them to fuse with the cell membrane and release their contents into the extracellular space.
This process, called exocytosis, is one of life’s most vital mechanisms. Without it, hormones couldn’t reach their target organs, neurons couldn’t communicate, and digestive enzymes wouldn’t reach the gut. The Golgi doesn’t just facilitate life; it empowers cells to participate in complex, multicellular systems. It transforms cells from isolated factories into integrated components of tissues, organs, and organisms.
A Role in Building the Cell’s Identity
While much of the Golgi’s work involves shipping and secretion, its influence extends to the very identity of the cell. One of its most crucial roles is in producing and modifying glycolipids and glycoproteins—molecules that stud the cell membrane and act as biological signatures.
These surface molecules are essential for cell-cell recognition, immune response, and tissue formation. For instance, the ABO blood group system depends on specific glycosylation patterns on red blood cell surfaces—patterns orchestrated in part by the Golgi apparatus. A small change in glycosylation can determine whether a blood transfusion is life-saving or deadly.
The Golgi is also instrumental in maintaining the polarity of epithelial cells, which have distinct “top” and “bottom” surfaces with different functions. This spatial organization is vital for processes like nutrient absorption in the gut or the filtration of waste in the kidney. By directing the traffic of proteins and lipids to specific membrane domains, the Golgi helps create the architecture of life.
A Dynamic and Responsive Organelle
Far from being a static stack of cisternae, the Golgi apparatus is a dynamic, responsive structure. Its shape, size, and organization can change depending on the needs of the cell. In some cells, it appears as a single, centralized stack; in others, it’s dispersed throughout the cytoplasm in mini-stacks. These changes can reflect developmental stages, stress responses, or disease states.
During mitosis—the process of cell division—the Golgi temporarily disassembles into vesicles and tubules, then reassembles in the daughter cells. This fragmentation ensures equal distribution and prevents interference with the mitotic spindle. It’s as if the Golgi takes a brief intermission, only to resume its duties with renewed vigor once division is complete.
The Golgi also responds to environmental cues, such as nutrient levels or cellular stress. In cancer cells, the Golgi is often enlarged or fragmented—a reflection of the increased demand for protein synthesis and secretion. Researchers now study Golgi morphology as a potential diagnostic marker for diseases ranging from Alzheimer’s to viral infections.
The Golgi and the Viral Hijack
In the perpetual arms race between host and pathogen, the Golgi apparatus is a coveted target. Many viruses exploit the Golgi’s trafficking system to replicate and spread. Some viruses enter the Golgi network to modify their surface proteins, using its enzymatic machinery for their own benefit. Others hijack vesicle trafficking pathways to exit the cell more efficiently.
For example, coronaviruses—including SARS-CoV-2—rely on the Golgi to process and package their spike proteins. These proteins must be correctly folded and glycosylated to facilitate viral entry into new host cells. Disrupting Golgi function can interfere with viral replication, making the organelle a potential target for antiviral therapies.
Yet, the Golgi is not defenseless. It can mount stress responses, recruit immune-related proteins, and even participate in innate immunity. In the quiet war that rages within our cells, the Golgi is both a tool and a battleground.
Cracks in the System: When the Golgi Fails
Like any complex machine, the Golgi apparatus is vulnerable to dysfunction. Mutations in genes that regulate Golgi enzymes or vesicle trafficking can lead to serious diseases—collectively known as congenital disorders of glycosylation (CDGs). These rare but devastating conditions can cause developmental delays, neurological deficits, immune dysfunction, and metabolic abnormalities.
One example is CDG type II, in which defects in Golgi glycosylation enzymes lead to improperly formed glycoproteins. These proteins, essential for brain development, immune signaling, and hormone function, can’t perform their roles correctly. There is no cure—only symptom management—and many children with severe forms of CDG die in early childhood.
Neurodegenerative diseases also show signs of Golgi disruption. In Alzheimer’s disease, for instance, neurons exhibit fragmented Golgi structures. The loss of Golgi integrity may contribute to protein misfolding and cellular stress, accelerating the disease process. Scientists are now exploring whether protecting Golgi function could offer new avenues for treatment.
Even in cancer, where cells grow uncontrollably, the Golgi is often reprogrammed to support rapid proliferation. Tumor cells rely on enhanced secretion and altered glycosylation to invade tissues and evade immune detection. Understanding how the Golgi is rewired in cancer may lead to therapies that cut off the tumor’s lifeline.
A Beacon for Future Discoveries
The Golgi apparatus is no longer the mysterious “internal reticular apparatus” glimpsed by Camillo Golgi more than a century ago. It is a dynamic, multifunctional hub whose importance grows with every new discovery in cell biology, immunology, virology, and medicine. Its fingerprints are found on nearly every process that sustains life, from digestion to immunity to memory.
As we move deeper into the molecular age—armed with CRISPR, cryo-electron microscopy, and single-cell sequencing—the Golgi remains a fertile ground for exploration. What governs its organization? How does it communicate with other organelles? Can we manipulate its pathways to treat disease or enhance immunity?
These questions are no longer the domain of academic curiosity. They are frontiers of medical science, with the potential to improve lives, heal diseases, and deepen our understanding of what it means to be alive.
The Humble Hero of the Cell
In a world obsessed with visible wonders—galaxies, whales, mountains—it’s easy to overlook the microscopic marvels within us. The Golgi apparatus, with its soft curves and silent labor, may never make headlines or grace posters in classrooms. But without it, our cells would be lost in chaos. Proteins would drift without purpose, hormones would miss their marks, and the fragile order of life would unravel.
The Golgi does not seek attention. It does not boast of its elegance or protest its invisibility. Yet in every heartbeat, every breath, and every thought, its presence is felt. It is the craftsman behind the curtain, the maestro of molecular traffic, the architect of cellular identity.
So the next time you marvel at the complexity of life—or the precision of science—spare a thought for the Golgi apparatus. For in its quiet chambers, life’s greatest symphony is composed and conducted every second of every day.