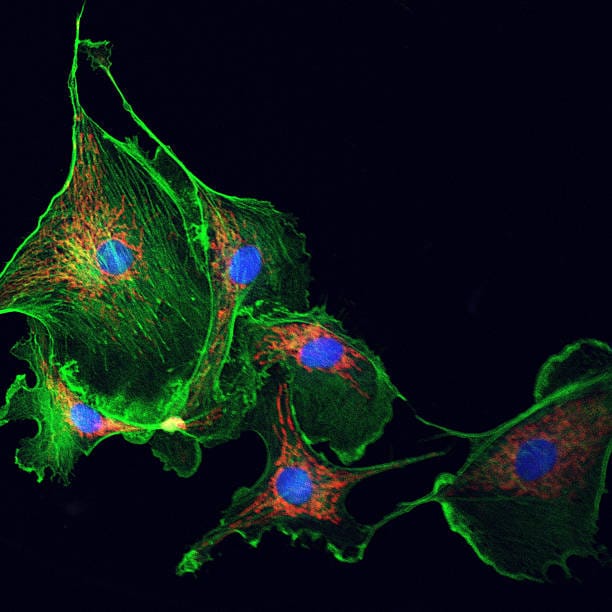
Beneath the surface of every living cell lies a world of astonishing complexity. Though they may appear as simple blobs under a microscope, cells are anything but chaotic. They possess a remarkable internal structure, a kind of invisible scaffolding that gives them shape, strength, and the ability to move, divide, and even communicate. This dynamic and intricate support system is known as the cytoskeleton.
Far from being a static frame, the cytoskeleton is alive with motion and change. It is a network of protein filaments that stretches across the cytoplasm, constantly assembling, disassembling, and reassembling as the cell grows, adapts, and responds to its environment. Without it, the cell would collapse into an inert blob, incapable of performing even the most basic tasks of life.
The cytoskeleton is not just essential for maintaining a cell’s shape—it is the architectural and logistical backbone of cellular life. It provides mechanical support, enables intracellular transport, drives cell division, powers locomotion, and helps cells sense and respond to their surroundings. In essence, the cytoskeleton is what turns the cell from a sack of chemicals into a living, responsive, self-organizing entity.
The Architecture of Cellular Stability
When people think of skeletons, they usually picture bones and joints—rigid, unchanging structures. But the cytoskeleton is profoundly different. It is composed of three primary types of protein filaments: microfilaments, intermediate filaments, and microtubules. Each plays a distinct role, and together they weave a dynamic mesh that supports the cell’s architecture and function.
Microfilaments, also known as actin filaments, are the thinnest of the three. Composed of the protein actin, these filaments form dense networks just beneath the plasma membrane. They are responsible for maintaining the cell’s shape and enabling movements such as crawling, contracting, and engulfing external particles. Actin is one of the most abundant and highly conserved proteins in all of biology, a testament to its fundamental importance.
Microtubules are the largest of the cytoskeletal filaments. They are hollow tubes made from tubulin proteins and function like highways inside the cell. They guide the movement of organelles, vesicles, and chromosomes during cell division. Microtubules also determine the positions of organelles and form the core of structures like cilia and flagella, which some cells use to move or to sense their environment.
Intermediate filaments are more stable and durable than the other two types. They provide tensile strength, helping cells resist mechanical stress. Unlike microfilaments and microtubules, which are composed of a single type of protein, intermediate filaments are made from a variety of proteins depending on the cell type—keratins in skin cells, vimentin in connective tissue, and neurofilaments in neurons. These filaments anchor organelles in place and create a resilient internal scaffold that reinforces cellular integrity.
Together, these three filament systems form a resilient and adaptable framework that shapes the cell and empowers it to perform its diverse tasks. But what’s truly remarkable is how these structures interact, overlap, and influence one another in a delicate dance choreographed by the cell’s needs and environment.
Building and Rebuilding: The Dynamic Nature of the Cytoskeleton
If you were to watch a time-lapse video of the cytoskeleton inside a living cell, you would witness something extraordinary. Filaments extend and retract, networks shift and flow, and entire structures assemble and vanish with astonishing speed. This constant remodeling is driven by the polymerization and depolymerization of the filament subunits—a process that is tightly regulated by cellular signaling pathways.
For instance, actin filaments can rapidly grow by adding actin monomers to one end (the “plus” end) while simultaneously losing subunits at the other end (the “minus” end), a phenomenon known as treadmilling. This allows cells to push their membranes forward during movement or change shape in response to stress. Similarly, microtubules exhibit dynamic instability—they alternately grow and shrink in cycles that are crucial for cell division and intracellular transport.
This dynamism is not chaotic but exquisitely coordinated. The cell uses dozens of accessory proteins to regulate filament growth, branching, crosslinking, and severing. Some proteins cap filament ends to prevent growth, others bind along the sides to stabilize structures, and still others sever filaments to promote turnover or reshape the network.
This balance between stability and flexibility is what gives the cytoskeleton its unique power. It can form long-lasting structures like the axons of neurons or the mitotic spindle during cell division, and yet it can also quickly dissolve and reassemble in response to signals. This makes it not just a structural support system, but an active participant in the life of the cell.
Cellular Highways and Molecular Motors
One of the most fascinating aspects of the cytoskeleton is its role in intracellular transport. Just as cities rely on highways and delivery trucks to move goods, cells depend on microtubules and actin filaments to transport proteins, organelles, and vesicles to where they are needed.
This movement is powered by molecular motor proteins—tiny machines that walk along the cytoskeletal filaments using energy from ATP. On microtubules, the two main types of motor proteins are kinesins and dyneins. Kinesins usually move cargo toward the periphery of the cell (the plus end), while dyneins move cargo inward (toward the minus end). On actin filaments, the most well-known motor protein is myosin, which is essential for muscle contraction but also plays roles in cargo transport and cell movement.
These molecular motors are marvels of biological engineering. Each step they take involves a carefully orchestrated cycle of ATP binding and hydrolysis, leading to changes in shape that propel them forward. Some motors carry vesicles packed with neurotransmitters along neuronal axons. Others help position mitochondria, move chromosomes during mitosis, or ferry newly synthesized proteins from the endoplasmic reticulum to the Golgi apparatus.
Without this system of intracellular logistics, cells would be unable to maintain their organization or function properly. Diseases such as Alzheimer’s and Huntington’s have been linked to defects in axonal transport, highlighting the vital importance of this system to cellular health.
Shape, Movement, and Metamorphosis
While the cytoskeleton gives cells their structural integrity, it also allows them to reshape themselves in profound ways. During embryonic development, cells must migrate, divide, and organize into complex tissues and organs—all actions that depend on the cytoskeleton.
One of the most dramatic examples of cytoskeletal behavior is cell migration. Whether in a developing embryo, an immune response, or wound healing, cells need to move toward specific signals. This movement is driven by actin polymerization at the leading edge of the cell, creating a protrusion called a lamellipodium. Adhesion molecules anchor this protrusion to the substrate, and myosin motors contract the rear of the cell, pulling it forward.
This crawling movement is orchestrated by signaling molecules like Rho GTPases, which control where and when actin filaments are assembled. The result is a cell that can sense its environment, choose a direction, and move purposefully—almost as if it had a will of its own.
But the cytoskeleton doesn’t just help cells move; it also transforms them. During cell division, microtubules form the mitotic spindle, which aligns and separates chromosomes. Actin and myosin form the contractile ring that pinches the cell in two. These feats of engineering must be executed with precision, or the results can be catastrophic—leading to aneuploidy, cancer, or cell death.
Even more stunning is the way the cytoskeleton enables certain cells to change their shape entirely. Immune cells can squeeze through tiny gaps between endothelial cells to reach sites of infection. Neurons extend long, slender axons and dendrites to form intricate networks. Epithelial cells form tight sheets with apical-basal polarity. All of this shape-shifting relies on the cytoskeleton’s capacity to remodel itself in response to internal plans and external cues.
The Cytoskeleton and Cellular Communication
Though it may not speak, the cytoskeleton is deeply involved in cellular communication. It helps transmit mechanical and chemical signals from the outside world into the cell’s interior, influencing gene expression, metabolism, and fate decisions.
One way this happens is through mechanotransduction—the process by which cells convert physical forces into biochemical signals. For example, when endothelial cells lining blood vessels sense the shear stress of flowing blood, they respond by reorganizing their cytoskeleton and activating specific genes. Similarly, when stem cells grow on stiff versus soft surfaces, they can differentiate into bone or fat cells depending on the mechanical properties of their environment—an effect mediated by the cytoskeleton.
Cytoskeletal elements are also closely tied to signaling pathways involving small GTPases, kinases, and phosphatases. When a receptor on the cell surface is activated, it can trigger changes in actin polymerization, leading to changes in shape, motility, or adhesion. These feedback loops allow the cell to dynamically adapt to its surroundings and to coordinate complex behaviors like chemotaxis, immune responses, and tissue development.
The cytoskeleton even plays a role in organizing the distribution of membrane proteins and signaling complexes, influencing how signals are perceived and processed. Far from being just an internal scaffold, it is a central integrator of the cell’s interactions with the world around it.
When the Framework Fails
As vital as the cytoskeleton is to life, it is also vulnerable. Defects in its components or regulators can lead to a wide range of diseases, collectively known as cytoskeletal disorders.
In cancer, for example, mutations in actin-binding proteins or motor proteins can promote uncontrolled cell movement and invasion. During metastasis, tumor cells hijack the cytoskeletal machinery to detach from their neighbors, migrate through tissue, and establish new colonies in distant organs.
Neurodegenerative diseases also frequently involve cytoskeletal dysfunction. In Alzheimer’s disease, the microtubule-associated protein tau becomes hyperphosphorylated and forms toxic tangles, disrupting transport in neurons. In amyotrophic lateral sclerosis (ALS), defects in axonal transport and intermediate filament organization contribute to motor neuron degeneration.
Even more rare are inherited cytoskeletal disorders like epidermolysis bullosa simplex, caused by mutations in keratin genes, which result in fragile skin that blisters easily. Or hereditary spherocytosis, where mutations in cytoskeletal proteins of red blood cells lead to anemia.
These conditions underscore the essential role of the cytoskeleton in maintaining not just cellular structure, but organismal health.
The Cytoskeleton in Evolution and Innovation
The cytoskeleton is not unique to humans—or even to animals. It is a deeply conserved feature of eukaryotic life, present in everything from yeast to plants to protozoa. In fact, even some bacteria possess primitive cytoskeletal elements like FtsZ and MreB, which are homologs of tubulin and actin. This suggests that the origins of the cytoskeleton stretch back billions of years, and that it was a key innovation in the evolution of cellular complexity.
In multicellular organisms, the cytoskeleton is central to the development of tissues and organs. It guides cells as they differentiate, align, and cooperate to build larger structures. It underlies the beating of cilia in respiratory epithelia, the contractions of muscle fibers, the growth of neurons, and the formation of blood vessels. It is the invisible hand shaping life from within.
Moreover, the cytoskeleton is a source of inspiration for bioengineers and nanotechnologists. Researchers are developing artificial cytoskeletons in synthetic cells, harnessing molecular motors to build nanoscale machines, and using cytoskeletal proteins to understand the principles of self-assembly and self-repair. The same properties that make the cytoskeleton so powerful in nature—its flexibility, responsiveness, and modularity—are now being harnessed in the laboratory to design the next generation of smart materials and medical devices.
The Inner Universe
To peer inside a cell is to glimpse an alien world—one governed by forces and structures that defy our ordinary intuitions. The cytoskeleton is the architecture of that world, the framework that makes cellular life possible. It is the internal compass that guides movement, the skeleton that holds everything together, and the unseen engineer that builds and rebuilds the cell from moment to moment.
Yet it is also a teacher. In its constant dance between order and chaos, structure and fluidity, the cytoskeleton offers a lesson in how life adapts, persists, and evolves. It reminds us that strength need not be rigid, that stability can coexist with change, and that even the most complex systems can arise from simple building blocks, given enough time and the right conditions.
As science continues to unravel the mysteries of the cytoskeleton, one thing remains clear: the cell is not a passive container of genetic information. It is an active, dynamic, and intelligent system, shaped by forces both visible and invisible. And at the heart of it all lies the cytoskeleton—a living architecture as elegant as it is essential.