Deep within the soil beneath our feet, inside the oceans, and even across our own skin, an ancient battle rages—a silent, invisible war between microscopic organisms. Bacteria, fungi, viruses, and other microbes engage in a constant struggle for survival, competing for space, nutrients, and dominance. In this microscopic melee, some organisms have evolved a remarkable weapon: the ability to produce antibiotics.
These naturally occurring compounds are not just chemical accidents. They are the tools of survival, crafted over millions of years through evolutionary pressure. Microbes manufacture antibiotics to kill or inhibit their rivals, ensuring their own persistence in crowded and hostile environments. What began as a biochemical turf war in nature has since become a cornerstone of modern medicine, saving millions of human lives.
The story of antibiotics is not just a tale of scientific discovery—it is a story of serendipity, of deep curiosity, and of nature’s ability to hide its most powerful gifts in the smallest corners of existence. At the heart of it all lies a stunning truth: the most potent weapons in our fight against disease are made by life itself.
From Molds to Miracles: The Birth of Antibiotics
The story of antibiotics began not in a gleaming laboratory, but in a messy, overlooked Petri dish. In 1928, Alexander Fleming, a Scottish bacteriologist working at St. Mary’s Hospital in London, returned from vacation to find that one of his staphylococcal cultures had been contaminated by a blue-green mold. Strangely, where the mold grew, the bacteria had disappeared. Fleming had stumbled upon Penicillium notatum, a common mold with an uncommon talent: it secreted a substance that killed bacteria.
This was penicillin—the first true antibiotic. It would take more than a decade before it could be isolated, mass-produced, and brought to the battlefield during World War II, where it saved countless soldiers from death by infection. But Fleming’s discovery was only the beginning. Scientists soon realized that the microbial world was a vast, untapped source of natural drugs.
Microbes, especially certain bacteria and fungi, turned out to be prolific chemists. They synthesized complex molecules—antibiotics—with astonishing precision. These molecules could target bacterial structures or processes in ways that human-designed chemicals could not match. With each new discovery, science peeled back a layer of nature’s pharmacopoeia.
Soil: Earth’s Hidden Apothecary
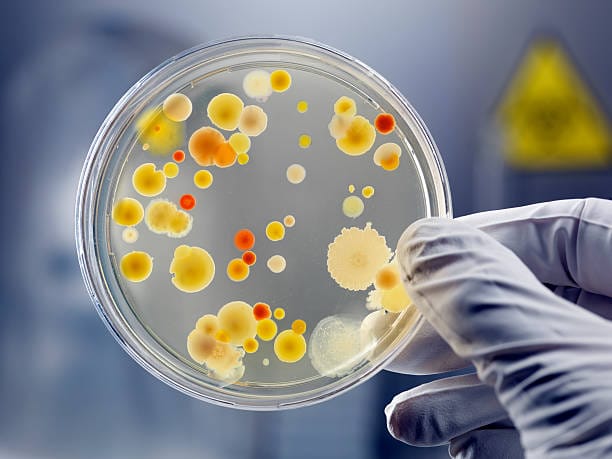
Most of the antibiotics used today originate from soil-dwelling bacteria, particularly a genus called Streptomyces. These microbes, abundant in forest floors, grasslands, and gardens, produce a dizzying array of bioactive compounds. Under the microscope, Streptomyces resemble tiny threads weaving intricate mycelial networks through the dirt. But chemically, they are powerhouses—capable of producing over two-thirds of all known antibiotics.
Why soil? Because it’s one of the most competitive environments on Earth. Microorganisms living there must battle for limited resources. In this microbial battlefield, antibiotic production provides a decisive advantage.
For decades, microbiologists have scoured the Earth’s soil for new antibiotic-producing organisms. Using a process known as “bioprospecting,” they collect samples from remote or extreme environments: deep caves, polar regions, deserts, and even ocean sediments. These samples are brought to the lab, cultured, and tested against pathogens. Occasionally, a microbe is found that produces a new antibiotic, opening the door to novel treatments.
The antibiotic streptomycin, for instance, was discovered in 1943 by Selman Waksman and his colleagues from Streptomyces griseus. It became the first effective treatment for tuberculosis and earned Waksman a Nobel Prize. Since then, dozens of other important antibiotics have been isolated from Streptomyces, including tetracycline, chloramphenicol, and erythromycin.
Microbial Chemistry: How Antibiotics Are Made
The ability of microbes to make antibiotics stems from their sophisticated genetic machinery. Within their DNA lie clusters of genes—biosynthetic gene clusters (BGCs)—responsible for assembling antibiotic molecules. These genes encode enzymes that perform step-by-step transformations, like a molecular assembly line, constructing antibiotics with exquisite control over structure and function.
Each enzyme in the pathway performs a specific reaction: adding a chemical group, forming a ring, inserting a sugar molecule, or modifying a backbone. The resulting molecule might inhibit bacterial ribosomes, block DNA replication, or puncture cell membranes. These precise biochemical tools are what make antibiotics so effective.
Many microbial antibiotics belong to classes such as β-lactams, macrolides, aminoglycosides, and glycopeptides. Each class has a different mode of action and spectrum of activity. For example:
- β-lactams (e.g., penicillin) disrupt bacterial cell wall synthesis.
- Aminoglycosides (e.g., gentamicin) interfere with protein production.
- Macrolides (e.g., erythromycin) bind to bacterial ribosomes and halt protein translation.
- Glycopeptides (e.g., vancomycin) block peptidoglycan formation in bacterial walls.
Remarkably, microbes don’t just produce a single compound. Many species can tweak their own biosynthetic pathways, generating related compounds called analogs. This chemical diversity is vital. It allows microbes to avoid developing resistance to their own products and gives researchers a broader palette for drug development.
The Role of Fermentation: Brewing Life-Saving Molecules
Once a promising antibiotic-producing microbe is discovered, the next step is scaling up. Microbial fermentation is the process by which microorganisms are grown in large tanks and induced to produce antibiotics in high quantities. The setup resembles a brewery, but instead of beer, it yields medicine.
Fermentation conditions must be optimized—temperature, pH, oxygen levels, and nutrient composition all influence the yield. Microbes are often fed sugars, nitrogen sources, and minerals to support rapid growth and metabolite production. In industrial fermenters, vast populations of bacteria or fungi churn in carefully controlled environments, their metabolic machinery harnessed for human benefit.
After fermentation, the antibiotic is extracted, purified, and tested for safety and efficacy. In many cases, the raw compound is chemically modified—semisynthetic antibiotics like amoxicillin are derived from natural penicillins but enhanced to improve stability, absorption, or spectrum of activity.
Over time, the process of fermentation has been refined with advances in biotechnology. Genetic engineering allows scientists to boost production yields, delete unwanted pathways, and create designer microbes capable of making novel antibiotics. The once-mysterious art of natural product fermentation has become a precise and powerful industrial science.
Antibiotic Resistance: A Race Against Evolution
As miraculous as antibiotics are, they come with a caveat: the more we use them, the faster bacteria evolve resistance. Resistance arises through mutations or the acquisition of resistance genes from other bacteria, often via plasmids—circular bits of DNA that can jump from one cell to another.
Antibiotic resistance is not a man-made phenomenon. In nature, microbes have been evolving resistance to one another’s compounds for billions of years. In fact, genes conferring resistance to penicillin and tetracycline have been found in 30,000-year-old permafrost samples. What is new, however, is the scale and speed of resistance today, driven by human overuse and misuse of antibiotics.
Modern agriculture exacerbates the problem. In many countries, antibiotics are routinely added to animal feed to promote growth and prevent disease. This creates reservoirs of resistant bacteria that can spread to humans through food, water, and the environment.
Hospitals, too, are hotspots for resistance. Bacteria like Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa have evolved multidrug resistance, making infections harder to treat and increasing mortality.
This growing crisis has revived interest in natural product discovery. Scientists now return to the microbial world, seeking out new organisms and untapped biosynthetic pathways. It’s a race against evolution, a search for compounds that can outwit bacterial defenses before the infections they cause become untreatable.
The Revival of Forgotten Microbes
In the last decade, a renewed focus on neglected and extreme environments has breathed new life into antibiotic discovery. Soil is no longer the only frontier. Scientists now explore marine sediments, thermal vents, salt flats, and even insect guts for rare microbes capable of producing novel drugs.
One breakthrough came from a technique called the iChip, which allows scientists to grow previously “unculturable” bacteria by mimicking their natural environment. Using this method, researchers discovered Eleftheria terrae, a microbe from a Maine soil sample that produces teixobactin—a powerful new antibiotic that shows promise against resistant pathogens and, intriguingly, seems less prone to inducing resistance.
The genome mining revolution has also opened new doors. Advances in DNA sequencing have revealed that many microbes harbor “silent” biosynthetic gene clusters—genetic blueprints for antibiotics that are not expressed under normal lab conditions. With the right stimuli or genetic tweaking, these hidden pathways can be activated, revealing new chemical treasures.
Even synthetic biology is playing a role. Scientists are now building artificial biosynthetic pathways in engineered microbes, combining genetic parts from different organisms to create “unnatural natural products.” This approach allows for the design of antibiotics with entirely new structures and modes of action.
The Future: Reimagining Microbial Medicines
The path forward lies not only in discovering new antibiotics, but also in understanding the ecology and evolution of microbial communities. Antibiotic production is just one facet of microbial life. By studying how microbes interact—with one another and with their hosts—scientists hope to develop smarter therapies.
This includes the use of probiotics to restore healthy microbiomes after antibiotic treatment, bacteriophages (viruses that infect bacteria) as precision killers of pathogens, and antimicrobial peptides that mimic natural immune defenses. It also means developing narrow-spectrum antibiotics that target specific pathogens while sparing beneficial bacteria—a kinder, more ecological approach to infection.
The use of microbes in making antibiotics is not a static achievement—it is a living, evolving relationship between humans and the microbial world. As we enter a new era of precision medicine and global health challenges, microbes may once again prove to be our greatest allies.
Conclusion: A Covenant With the Microbial World
The story of antibiotics is one of wonder, warning, and hope. From the chance observation of mold in a Petri dish to the industrial fermentation of lifesaving drugs, microbes have shown us that nature is not just a source of life—it is also a source of healing.
Yet this relationship demands respect. The overuse of antibiotics, the neglect of microbial ecology, and the slow pace of new drug discovery have pushed us to the edge of a post-antibiotic world. But if history teaches us anything, it is that the answers often lie beneath our feet—or inside the tiniest living things.
Microbes are ancient, adaptive, and endlessly inventive. As long as we continue to explore, to question, and to cherish the invisible engines of the biosphere, we may continue to find new antibiotics and new ways to fight disease. In doing so, we renew a sacred covenant: that by understanding nature, we can protect life—not just our own, but all life that depends on the delicate balance of the microbial world.