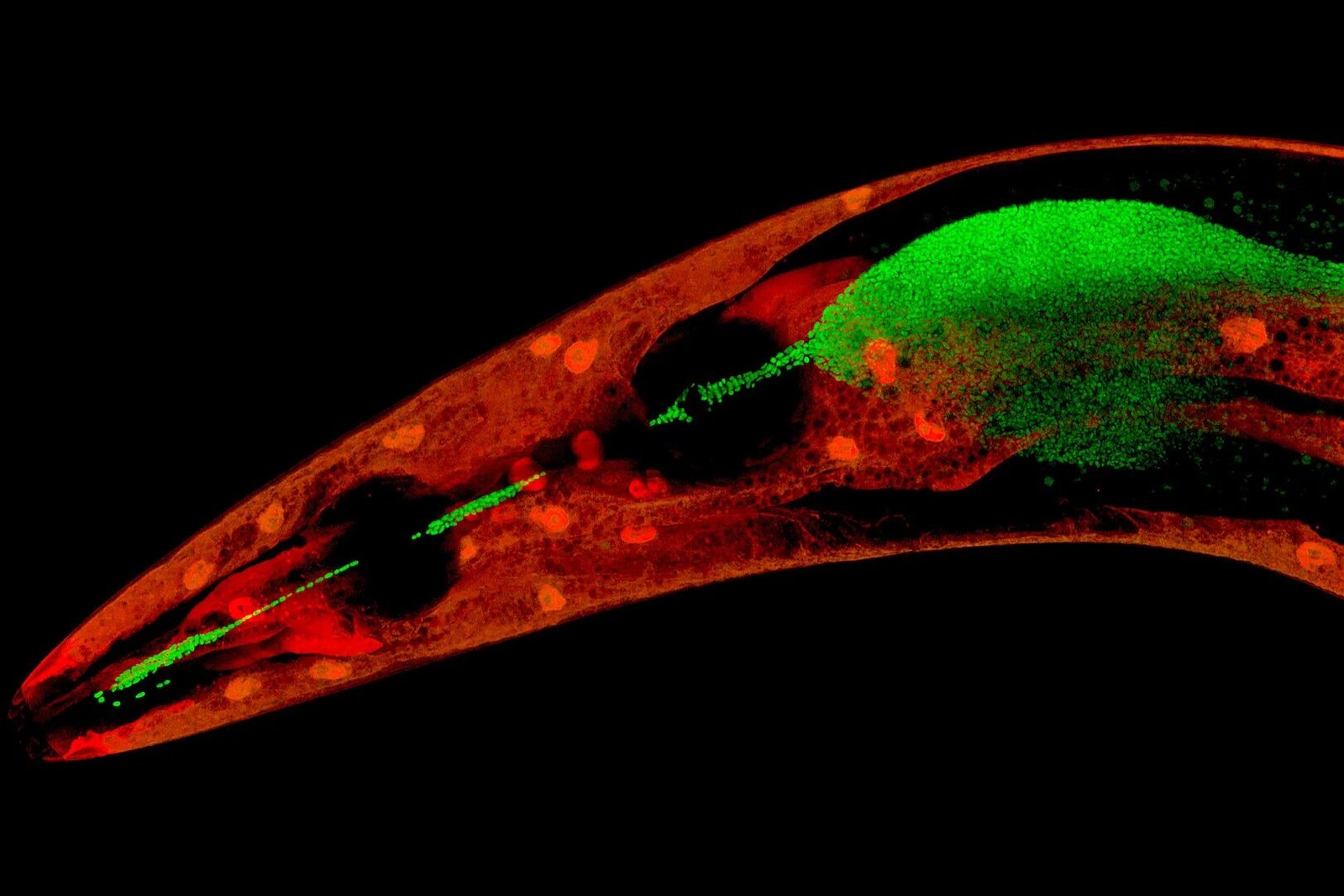
Inside the gut of a tiny, transparent worm called Caenorhabditis elegans, a hidden drama unfolds every day—a molecular dance between host and microbe, warfare and protection, survival and destruction. For years, scientists have known that the microbiome—the vast community of bacteria, viruses, and fungi living in and on all multicellular organisms—plays a crucial role in health and disease. But now, researchers at Kiel University have uncovered something astonishing: certain bacteria don’t just live peacefully inside their host—they actively help it fight off deadly infections using molecules no one expected them to make.
In a new study published in Nature Communications, researchers from the Collaborative Research Center (CRC) 1182, in collaboration with the Max Planck Institute for Terrestrial Microbiology and the University of Edinburgh, discovered that a species of Pseudomonas bacteria living in C. elegans produces sphingolipids—fat-like molecules once thought to be the exclusive domain of more complex life. And more importantly, these lipids appear to boost the worm’s defense system, shielding it from a lethal pathogen.
This is not just a footnote in microbiology. It may be the first glimpse into a vast, unseen mechanism of natural defense—one that could change how we treat infections and understand our own gut microbiomes.
The Mystery of the Microbial Bodyguard
For years, Dr. Katja Dierking and her team at Kiel University have used C. elegans as a model organism to study the intricate relationship between hosts and their microbiomes. The simplicity of the worm—just a millimeter long and fully transparent—makes it an ideal testbed for understanding how microbes and multicellular organisms co-evolve.
Back in 2019, the team revealed that certain Pseudomonas bacteria in the worm’s gut could protect it from infections. But no one knew how. What substance or strategy did the bacteria use to shield their host? The answers lay buried in the genetics and chemistry of an unassuming microbe.
Now, in this latest study, the team followed a complex web of clues—from gene clusters to lipid profiles—and uncovered a striking mechanism: Pseudomonas fluorescens was synthesizing sphingolipids, a rare kind of molecule in the bacterial world, but one that plays key roles in membrane structure and immune signaling in eukaryotes like us.
A Bacterial Secret Weapon Revealed
Sphingolipids are typically made by animals and plants, not bacteria. They help shape cell membranes, relay important cellular signals, and even determine how cells respond to stress. Until now, scientists believed only a few specialized bacterial groups could produce them—and Pseudomonas wasn’t on that list.
That changed when Dierking’s team discovered that P. fluorescens uses an alternative metabolic pathway to make sphingolipids, one never before documented in this genus. At the heart of this discovery was a unique biosynthetic gene cluster—a set of genes working together like an assembly line to manufacture the lipid molecules.
Through a blend of metabolic and transcriptional analyses, mass spectrometry, and even single-molecule studies, the team confirmed the unexpected: these bacterial sphingolipids weren’t random byproducts. They played a direct role in protecting the worm’s intestinal lining from the destructive effects of pathogens—specifically from Bacillus thuringiensis, a bacterium whose toxins punch holes in host cells.
Lipid Armor Against Microbial Assault
The protective effect wasn’t brute force. These lipids didn’t kill the invading pathogen. Instead, they strengthened the host’s own cellular defenses. When C. elegans was infected with B. thuringiensis, the toxins created tiny pores in the intestinal cell membranes, making the worm vulnerable to further infection. But with the help of the sphingolipids from P. fluorescens, the worm’s own lipid metabolism appeared to shift—fortifying cell membranes and making it harder for the pathogen to break through.
“It’s almost like the bacteria are helping the host reinforce its walls,” said Dr. Lena Peters, first author of the study and a researcher in Dierking’s group. “It’s a cooperative defense strategy, one we don’t usually see in this context.”
In other words, the bacteria weren’t just living in the gut; they were acting as chemical allies, arming their host with the tools to fight back.
A Microbial Gift with Ancient Roots
To trace the origins of this molecular defense, the team dug deeper into the genes responsible. They found that the biosynthetic gene cluster used by P. fluorescens to produce sphingolipids isn’t unique. Similar clusters exist in other host-associated gut bacteria, suggesting this ability might be far more widespread than previously believed.
That opens the door to a thrilling possibility: many animals—possibly including humans—may harbor bacteria that produce protective sphingolipids in their guts.
“It’s exciting to be authors on this important, breakthrough paper,” said Professor Dominic Campopiano from the University of Edinburgh. “We are pleased that our expertise in bacterial sphingolipid research has helped uncover a new role for these enigmatic lipids in the microbiome.”
The implications go beyond C. elegans. If similar interactions are happening in the human gut, understanding them could offer entirely new approaches to preventing or treating infections, especially in an age of rising antibiotic resistance.
From Worms to Humans: A New Frontier in Gut Health
This discovery fits into a broader scientific shift: the realization that our microbiome isn’t just a passive community—it’s an active partner in our health. The gut microbiome helps digest food, produces essential vitamins, and now, it seems, might also contribute directly to immune defense by manufacturing molecules once thought to be our exclusive domain.
“The fact that microbial metabolites like sphingolipids can support host defense against pathogens expands our understanding of how microbiomes protect us,” said Dierking. “It also hints at a future where we can design better treatments for microbiome-related diseases—not just by killing harmful bacteria, but by encouraging helpful ones.”
The research team, working under Kiel University’s Kiel Life Science (KLS) research initiative, hopes this deeper knowledge of host-microbiome interactions will lead to new therapeutic strategies—from strengthening the gut barrier in inflammatory diseases to designing probiotics that actively produce protective molecules.
The Invisible Guardians Within
What this study ultimately reveals is a story of cooperation on the smallest scale. In a world dominated by competition and survival, it’s easy to forget that some of our greatest allies are microscopic organisms working quietly inside us.
The worms may be tiny. The bacteria even smaller. But the message they send is enormous: health isn’t just something we build—it’s something we share.
Reference: Lena Peters et al, Polyketide synthase-derived sphingolipids mediate microbiota protection against a bacterial pathogen in C. elegans, Nature Communications (2025). DOI: 10.1038/s41467-025-60234-1