The human brain is a universe of complexity. Among its countless regions, the anterior insular cortex (aIC) stands out as a hub of emotional regulation, decision-making, and the integration of bodily sensations. It is the place where feelings meet awareness, where bodily signals shape our choices, and where the raw texture of experience becomes meaningful.
For years, neuroscientists have known that this region is implicated in neuropsychiatric conditions such as autism spectrum disorder (ASD) and depression—two disorders that profoundly affect how people experience the world. But one crucial question has remained: how does the aIC contribute to the unusual patterns of thought, emotion, and behavior seen in these conditions?
A new study from researchers at Tsinghua University, published in Molecular Psychiatry, offers a groundbreaking glimpse into this mystery. Their findings suggest that tiny immune cells in the brain, called microglia, may play a far more central role than previously imagined.
The Unsung Heroes of the Brain
Microglia are often described as the brain’s immune guardians. They clear away damaged cells, fight off pathogens, and help maintain balance in the nervous system. But their role is not limited to defense—they also shape the way neurons connect, prune synapses during development, and help regulate brain circuits that govern behavior.
If neurons are the “wires” of the brain, microglia are the caretakers, constantly fine-tuning the system. Yet, when microglia malfunction, the consequences can ripple through the entire network. The Tsinghua University team suspected that these cells might hold the key to understanding why the aIC is so deeply tied to ASD and depression.
A Tale of Two Mouse Models
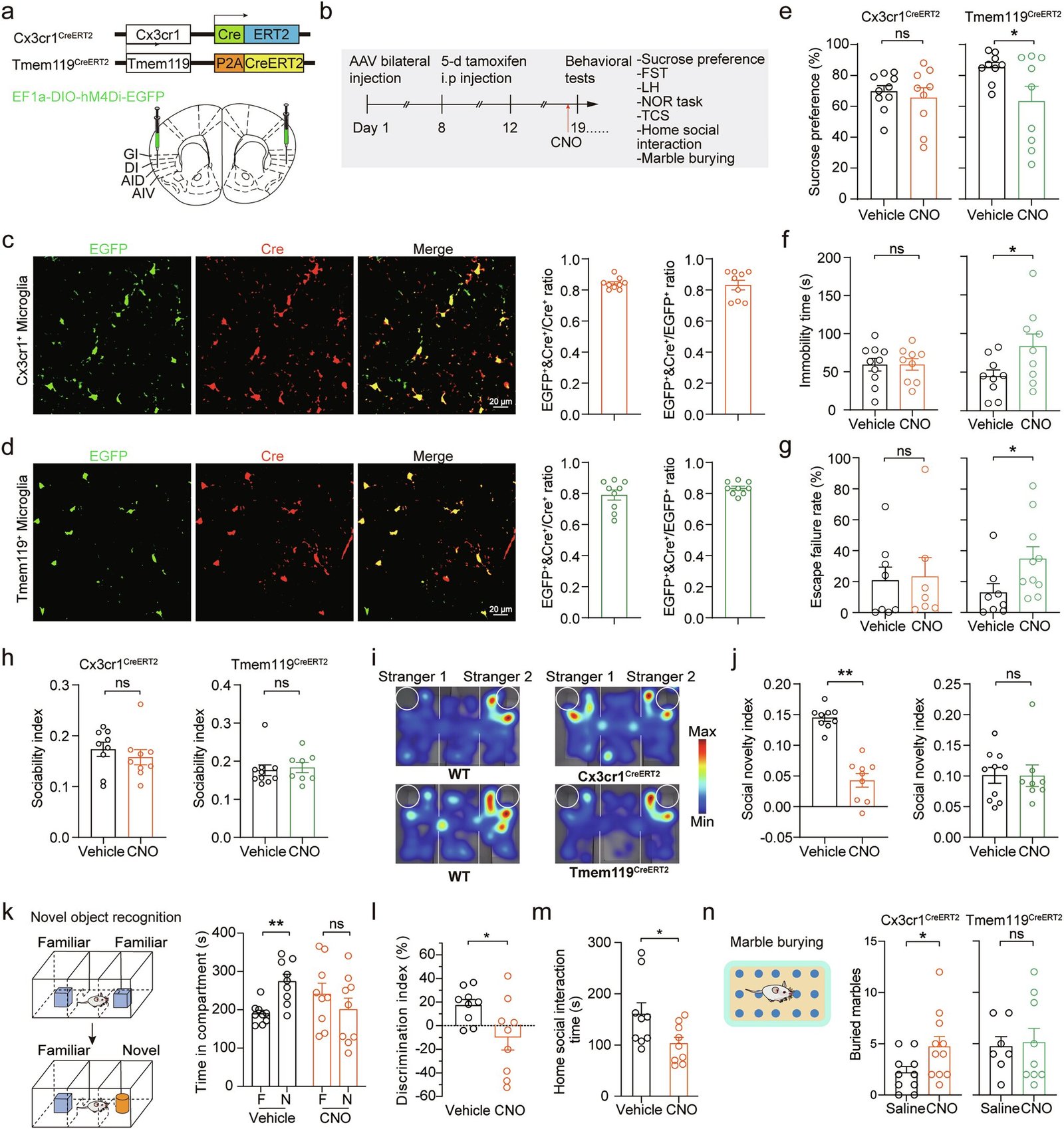
To test this idea, the researchers turned to two well-established mouse models.
The first group consisted of Cntnap2-deficient mice, genetically engineered to lack a gene known to be associated with autism in humans. These mice displayed behaviors reminiscent of ASD, such as social difficulties and repetitive actions.
The second group was subjected to chronic social defeat stress (CSDS), a protocol in which smaller mice are repeatedly exposed to larger, aggressive mice. Over time, the stressed mice developed symptoms resembling depression, including social withdrawal and a lack of interest in pleasurable activities.
By examining the brains of these two groups, the scientists were able to ask a critical question: Do microglia in the anterior insular cortex differ between autism-like and depression-like behaviors?
Two Subtypes, Two Stories
The answer was striking. The researchers identified two distinct subtypes of microglia that seemed to drive the behaviors observed in the mice.
- In the autism-like mice, Cx3cr1+ microglia showed abnormal shapes and functions. These changes were linked to the mice’s social deficits and repetitive behaviors.
- In the depression-like mice, Tmem119+ microglia appeared disrupted. Their dysfunction was tied to classic signs of depression in mice, such as reduced interest in sweetened water (a measure of pleasure) and poor performance in a forced swim test.
Not only did these microglia look different under the microscope, but they also behaved differently on a molecular level. Their gene expression patterns, electrophysiological properties, and effects on nearby neurons were distinct, suggesting that each subtype plays a unique role in shaping brain function and behavior.
Secret Signals and Molecular Clues
Digging deeper, the researchers used advanced proteomic and metabonomic analyses to identify molecules secreted by these microglia. Two stood out:
- Fbl, linked to the dysfunction of Cx3cr1+ microglia in autism-like mice.
- Hp1bp3, tied to the problems seen in Tmem119+ microglia in depression-like mice.
When these molecules were experimentally manipulated, the behavioral symptoms in the mice changed, confirming that they were central to the story.
This discovery suggests that microglia don’t just passively fail in ASD or depression—they actively send out faulty signals that reshape brain circuits and behavior.
Why This Matters
The implications of this work are profound. For decades, most research on psychiatric disorders has focused on neurons—the cells that transmit electrical signals in the brain. But this study highlights the importance of non-neuronal cells, showing that microglia may be equally critical in shaping mental health.
If similar findings are confirmed in humans, they could open the door to entirely new treatments. Instead of broadly targeting neurotransmitters, as most current drugs do, future therapies might focus on restoring balance to specific microglial subtypes, or even neutralizing the harmful molecules they release.
For individuals living with ASD or depression, this could mean more precise and effective interventions—treatments that address root causes rather than just symptoms.
The Road Ahead
Of course, this research is still in its early stages. The experiments were conducted in mice, and translating findings to humans is never straightforward. The human brain is vastly more complex, and ASD and depression are highly heterogeneous conditions influenced by genetics, environment, and life experience.
Yet, the study provides a roadmap for future exploration. If different microglia populations contribute to different disorders, then mapping these cells across various brain regions could revolutionize psychiatry. Researchers may one day identify microglial signatures for anxiety, schizophrenia, or bipolar disorder—turning once-mysterious illnesses into biologically grounded conditions with clear therapeutic targets.
A New Perspective on Mental Health
What makes this discovery so compelling is not just its scientific novelty, but its emotional resonance. It reminds us that behind every struggle with depression, every difficulty in social connection, and every repetitive behavior lies a deeply biological story.
The suffering of individuals with ASD and depression is not a failure of willpower, not a character flaw, but a reflection of intricate cellular processes playing out in the brain. The idea that something as small as a microglial cell could shape such profound human experiences is both humbling and hopeful.
Science is telling us, once again, that understanding the brain requires looking beyond the obvious. Sometimes the answers to life’s deepest challenges lie not in the grand scale of neurons and networks, but in the quiet work of microscopic caretakers, hidden deep within the folds of the cortex.
Conclusion: Tiny Cells, Vast Possibilities
The study from Tsinghua University offers a powerful glimpse into the hidden biology of autism and depression. By uncovering the roles of Cx3cr1+ and Tmem119+ microglia in shaping distinct behaviors, the researchers have illuminated a new frontier in neuroscience—one where immune cells and mental health are inseparably linked.
The journey from mice to medicine will be long, but the path is clearer than before. If future research confirms these findings in humans, it could transform the way we understand and treat neuropsychiatric disorders.
For now, we are left with a sense of awe: that in the tiny microglia of the anterior insular cortex, entire worlds of emotion, perception, and behavior may be written.
More information: Qiao-Ming Zhang et al, Anterior insular cortex regulates depression-like and ASD-like behaviors via the differential contribution of two subsets of microglia, Molecular Psychiatry (2025). DOI: 10.1038/s41380-025-03139-1.