When a patient walks into a hospital complaining of double vision, the last thing they expect is to be told they might have a brain tumor. For doctors, this kind of symptom raises immediate red flags. In one recent case, an MRI scan revealed a tumor nestled deep within the brain, in a location that made surgical biopsy highly dangerous. The usual route to a definitive diagnosis—cutting into the brain to extract tissue—could itself be life-threatening. What options remain when surgery is too risky and time is running out?
At Charité—Universitätsmedizin Berlin, one of Europe’s leading university hospitals, a team of researchers has devised a solution that sounds like something straight from science fiction but is already proving transformative in the real world. The answer? An artificial intelligence model capable of diagnosing brain tumors without the need for invasive procedures. By interpreting the molecular “fingerprints” of tumors, gathered from fluid samples instead of physical tissue, the AI model offers both speed and extraordinary accuracy. Welcome to the future of oncology diagnostics—precise, personalized, and powered by machine learning.
Beyond the Scalpel: The Need for a New Diagnostic Approach
For decades, diagnosing brain tumors has required a high-stakes procedure: open-brain surgery or needle biopsy to extract a sample, followed by days of pathology work to examine tissue under a microscope. It’s a gold standard that works well—until it doesn’t. In cases where tumors reside in inaccessible or dangerous regions of the brain, biopsies may risk severe complications, including paralysis, hemorrhage, or death. Some patients may not be eligible for surgery at all due to age, comorbidities, or the aggressive location of the tumor.
This diagnostic dilemma is more than just a logistical problem; it has profound implications for treatment. Cancer therapies today are no longer one-size-fits-all. Each tumor is a complex biological system, characterized by its tissue structure, molecular behavior, and genetic mutations. From the outside, tumors may look similar in imaging scans, but under the microscope—or deeper, at the genetic level—they can be vastly different. Correctly identifying the tumor subtype is essential for choosing the right therapy. A misdiagnosis can result in delayed or ineffective treatment and may even shorten a patient’s life expectancy.
Charité’s research team, led by Dr. Philipp Euskirchen and Dr. Sören Lukassen, believed it was time to look beyond the microscope and even beyond the traditional concept of tumor biology. What if the answer to safer, faster cancer diagnostics wasn’t in the tissue at all—but in the genetic material that tumors shed into bodily fluids?
Cracking the Code: Tumors Leave Molecular Clues Behind
Every cell in the body, including cancer cells, contains a genome—our biological blueprint made of DNA. But even more telling than the genes themselves are the ways those genes are regulated. This is where epigenetics comes in. Epigenetic modifications act like molecular switches, turning genes on or off depending on a cell’s environment and state. These modifications include methylation patterns, histone changes, and other chemical tags that influence how DNA is read and interpreted.
In cancer cells, these epigenetic patterns are dramatically altered. They’re not random, either. Tumors exhibit consistent and characteristic changes that act as molecular fingerprints. By examining these fingerprints, researchers can not only detect whether cancer is present but also determine the specific type and even subtype of the tumor.
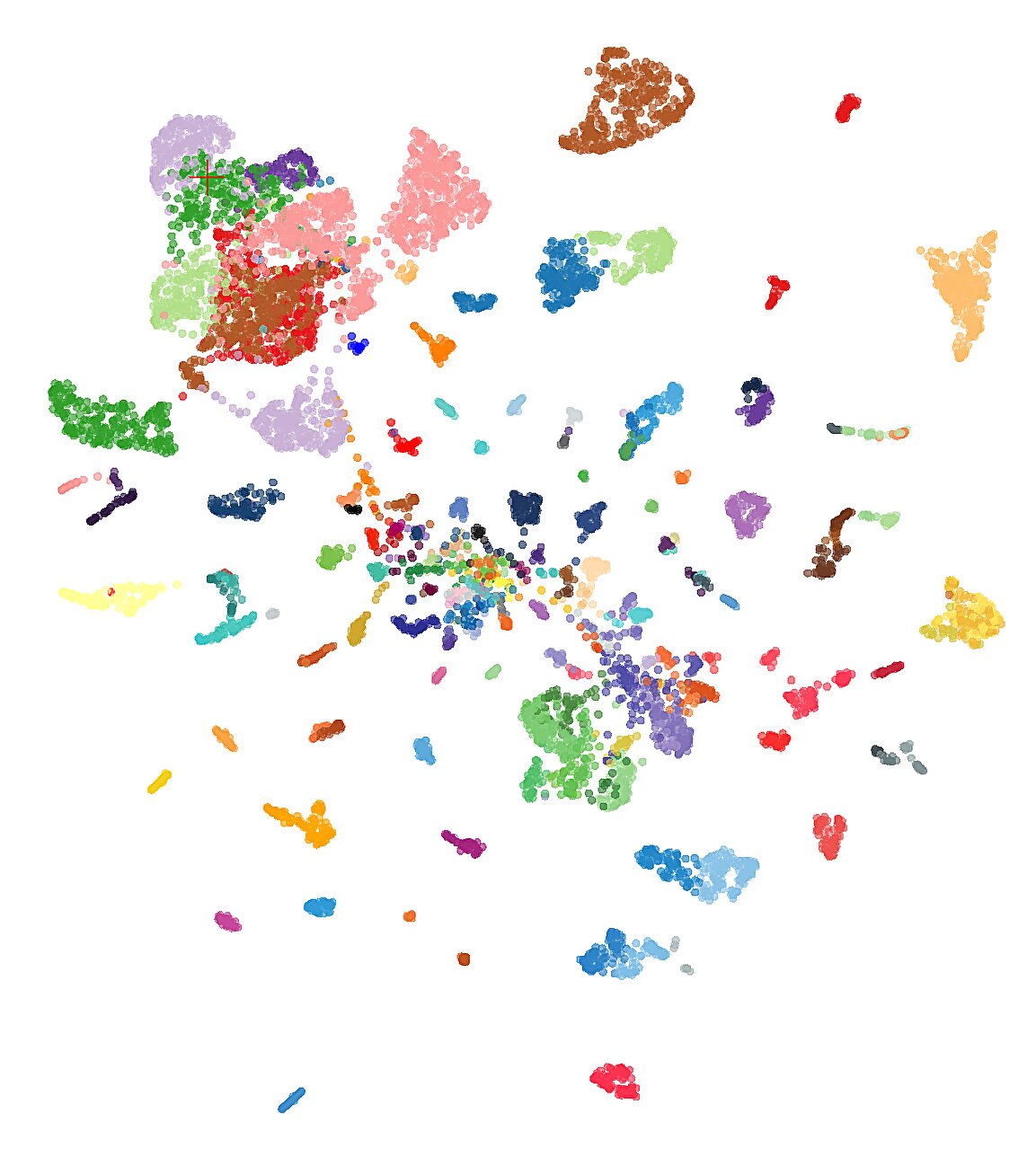
Dr. Euskirchen describes these patterns as “unmistakable fingerprints”—unique signatures encoded in the epigenome that offer far more information than the naked eye could ever detect. With hundreds of thousands of such modifications present in a single sample, traditional analysis becomes nearly impossible. That’s where artificial intelligence enters the picture.
Introducing crossNN: The AI That Thinks Like a Molecular Detective
At the core of this breakthrough is a new AI model known as crossNN, which stands for cross-neural network. While the name may sound technical, its concept is elegantly simple. The model was trained on thousands of known tumor types, each with a detailed molecular fingerprint. Once trained, it can then take a new, unknown fingerprint—perhaps from a patient’s cerebrospinal fluid or a small blood sample—and match it to the closest known tumor types with extraordinary precision.
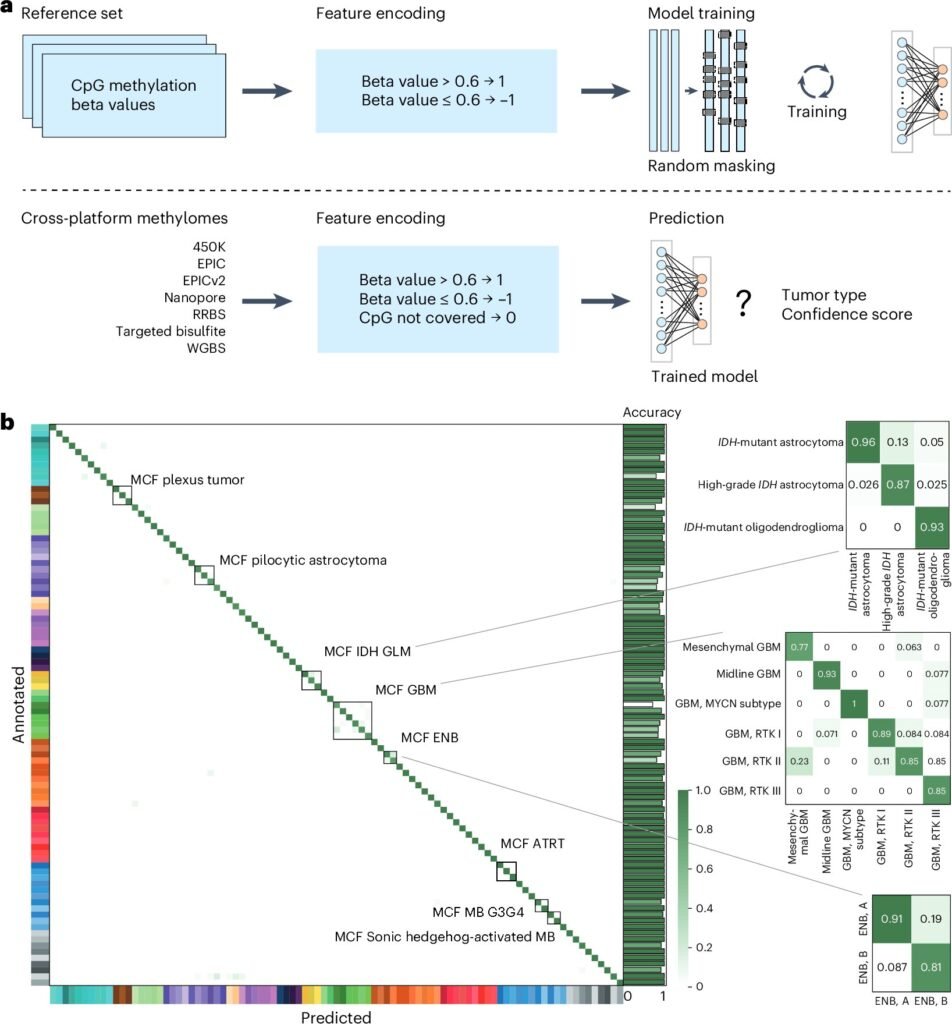
The training process was immense. The team fed crossNN data from over 5,000 tumors, each annotated and sequenced using a variety of techniques. Unlike previous AI models that struggled when input data came from different sequencing technologies or had incomplete information, crossNN was designed to be robust, flexible, and, crucially, explainable.
Explainability is the Achilles’ heel of many AI systems, especially in medicine. Doctors need to understand why a model reaches a certain diagnosis before they can trust it. The beauty of crossNN lies in its straightforward neural network structure, which avoids the “black box” trap and allows researchers to trace how each decision was made.
And the results? Nothing short of stunning. crossNN correctly diagnosed brain tumors in 99.1% of cases. When extended to tumors across all organ systems—more than 170 different types—the model still achieved a remarkable 97.8% accuracy.
Diagnosis from a Drop: The Power of Liquid Biopsy
Perhaps the most compelling part of this innovation is the way in which tumor DNA is collected. In traditional diagnostics, tissue biopsies remain the cornerstone. But crossNN allows for what’s known as a liquid biopsy, where cancerous DNA floating in fluids such as cerebrospinal fluid or blood is sampled and analyzed.
For brain tumors, cerebrospinal fluid (CSF) is especially valuable. The process of extracting CSF is much safer than brain surgery and involves a lumbar puncture—a relatively routine procedure. Once the fluid is obtained, a novel sequencing technology called nanopore sequencing is used to rapidly scan for epigenetic modifications.
This technique was used in the case of the double-vision patient mentioned earlier. Rather than risking a dangerous surgical biopsy, doctors extracted CSF and analyzed it using nanopore sequencing. crossNN immediately identified the tumor as a central nervous system lymphoma—a diagnosis that would have taken much longer and carried far greater risks using conventional methods. With this knowledge, doctors were able to initiate appropriate chemotherapy almost immediately.
Cancer Is Not a Monolith: The Importance of Tumor Subtypes
The days when cancers were classified solely by their organ of origin are long gone. Today, we understand that each tumor has its own molecular identity. For instance, breast cancer is not one disease—it is many, ranging from hormone-sensitive to HER2-positive to triple-negative subtypes, each with vastly different treatment strategies.
The same is true for brain tumors, where categories like glioblastoma, medulloblastoma, and oligodendroglioma vary in aggressiveness and treatment options. Mistaking one for another can lead to catastrophic treatment decisions.
crossNN doesn’t just detect the presence of a tumor; it identifies its subtype by comparing its epigenetic fingerprint against a vast database. In many cases, this level of granularity can mean the difference between a standard chemotherapy protocol and an opportunity to participate in a precision medicine clinical trial.
From Lab to Clinic: The Road Ahead for crossNN
Despite its extraordinary promise, crossNN is not resting on its laurels. The research team is moving quickly to bring the model into routine clinical use. Clinical trials are already being organized in collaboration with the German Cancer Consortium (DKTK), which includes eight research centers across the country.
These trials will assess the model’s performance not only in laboratory settings but also in real-world clinical environments, including intraoperative use—meaning diagnosis while the patient is still on the operating table. If successful, this could change how surgeries are planned and executed, allowing surgeons to adapt in real time based on AI feedback.
There are also plans to integrate crossNN into early cancer screening protocols, particularly for individuals with high genetic risk. Instead of waiting for symptoms or visible tumors, doctors could potentially detect cancer at a molecular level months or even years before it becomes dangerous.
Personalized Medicine’s New Partner
The concept of personalized medicine has long been a goal in oncology. Instead of treating every patient the same, therapies are tailored to the individual characteristics of each tumor. But personalized medicine requires personalized diagnosis, and that’s precisely where crossNN excels.
By incorporating multiple data types—from different sequencing technologies, from fluid and tissue samples, and from patients of all backgrounds—crossNN becomes a diagnostic Swiss Army knife. Its simplicity, accuracy, and adaptability make it ideal for use in both specialized research hospitals and smaller clinical centers.
Perhaps most importantly, its success shows that AI doesn’t need to be opaque or overly complex to be powerful. In fact, crossNN’s simplicity is one of its greatest strengths. It can be audited, trusted, and most importantly, understood by the very doctors who will rely on it to make life-saving decisions.
A Glimpse Into the Future
We are standing at the brink of a diagnostic revolution. With models like crossNN leading the way, the era of invasive biopsies and uncertain tumor classification may soon give way to fast, safe, and incredibly precise molecular diagnostics.
The patient with double vision was just the beginning. In the years to come, countless lives may be extended—or even saved—because a few drops of fluid, a bit of sequencing, and an intelligent algorithm could see what even the most skilled surgeon or pathologist could not.
At its core, the story of crossNN is a story of innovation born from necessity. It’s about replacing fear with information, delay with action, and invasive procedures with compassionate precision. For patients, that means less suffering, quicker answers, and better outcomes. For medicine, it means the dawn of a new paradigm where AI and biology walk hand in hand.
Reference: Dongsheng Yuan et al, crossNN is an explainable framework for cross-platform DNA methylation-based classification of tumors, Nature Cancer (2025). DOI: 10.1038/s43018-025-00976-5