In a remarkable medical first, scientists from Uppsala University Hospital have reported that genetically engineered islet cells from a donor pancreas survived for 12 weeks in a human patient with long-standing type 1 diabetes—without the need for any immunosuppressive drugs. The findings, published in the New England Journal of Medicine, open a new frontier in the pursuit of a long-sought dream: curing type 1 diabetes without trading one health burden for another.
This pioneering clinical trial, led by a Swedish team of endocrinologists, transplant surgeons, and immunologists, tested whether human donor cells—carefully edited to escape immune detection—could survive and function inside a living body, unaided by the toxic regimens traditionally required to prevent organ rejection. The answer appears to be yes.
The Quiet Struggle Behind Type 1 Diabetes
For millions of people around the world, life with type 1 diabetes is a relentless balancing act. The disease, which typically strikes in childhood or adolescence, occurs when the immune system destroys insulin-producing beta cells in the pancreas. Without insulin, blood glucose spirals out of control. Before insulin therapy was discovered in the 1920s, the condition was fatal.
Today, patients survive and even thrive with daily insulin injections or pumps, but the burden is immense. Fluctuating blood sugar, constant monitoring, strict diet, and a daily fear of hypoglycemia define the lives of those affected. Even with near-perfect management, people with early-onset type 1 diabetes face reduced life expectancy, higher cardiovascular risk, and a host of long-term complications—neuropathy, kidney disease, blindness.
Attempts to replace destroyed beta cells have been ongoing for decades. Islet transplants from deceased donors have offered brief glimmers of insulin independence—but always at a cost. The immune system sees foreign islets as invaders, just like it does viruses or bacteria. To prevent rejection, patients must take powerful immunosuppressive drugs that leave them vulnerable to infections and increase the risk of cancer, kidney failure, and more.
In short, the treatment has often seemed worse than the disease. Until now.
Engineering a Cloak of Invisibility: The Science Behind the Trial
In the new study, scientists didn’t try to trick the immune system—they rewrote the rules of engagement.
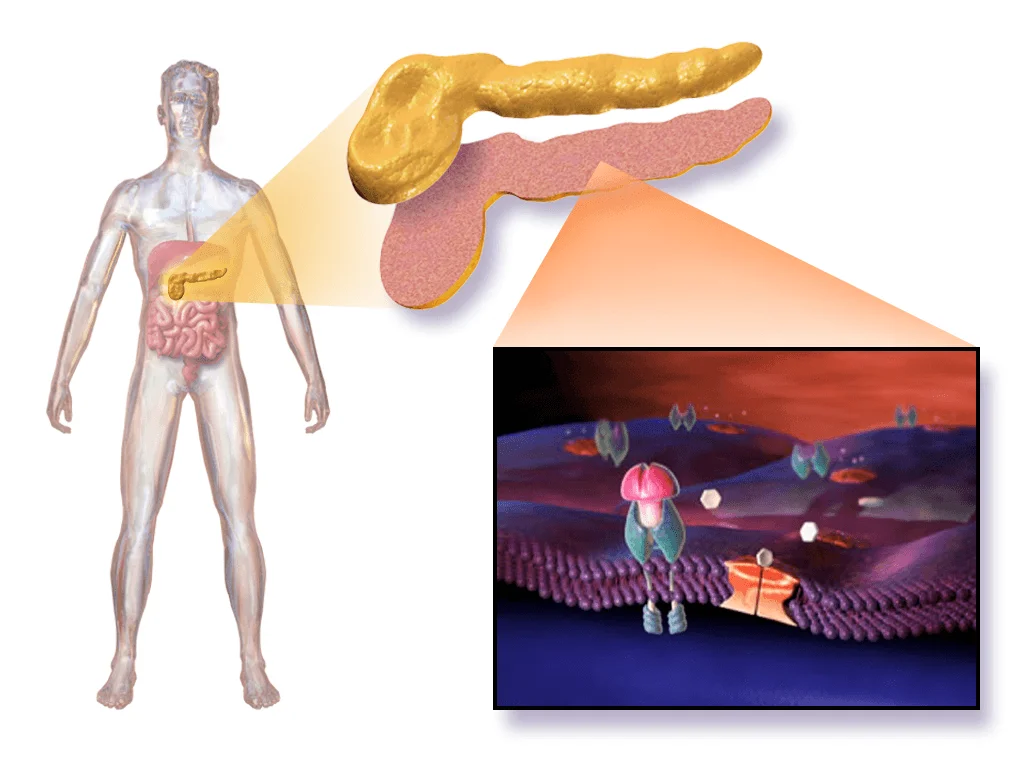
The research focused on a single patient: a 42-year-old man who had lived with type 1 diabetes for 37 years. Into his left forearm muscle, researchers transplanted a small quantity—just 7% of a full therapeutic dose—of genetically edited beta-cell clusters made from donor islets.
The procedure was a marvel of bioengineering. First, researchers harvested islet cells from a deceased organ donor who was blood-type O, ensuring compatibility. The islets were then dissociated into individual cells and genetically edited using CRISPR-Cas12b, a precision gene-editing tool.
Two critical genes—B2M and CIITA—were knocked out. These genes code for proteins essential to the cell’s visibility to the immune system. Without them, the donor cells could no longer display the molecular “ID cards” that typically alert T cells to destroy foreign invaders. But that wasn’t all.
The edited cells were also engineered to overexpress CD47, a molecule sometimes called the “don’t eat me” signal. This protein tells immune cells—especially macrophages and natural killer cells—to leave the cell alone. Together, these modifications created hypoimmune cells—a stealth version of the original islet cells designed to go undetected in hostile territory.
Finally, the cells were reclustered into pseudo-islets and injected into 17 tracks of the left brachioradialis muscle under general anesthesia. Crucially, the patient received no immunosuppressants, no steroids, no anti-inflammatory agents of any kind. His immune system remained fully intact.
The Proof Is in the Survival—and the Sugar
Over the course of 84 days, the scientists closely monitored the transplanted cells using blood tests, glucose tolerance assays, PET scans, and MRI imaging.
The results were nothing short of groundbreaking.
The unedited cells that remained—those not modified with the hypoimmune strategy—were attacked. The patient’s immune system mounted a full response: T cells swarmed, antibodies formed, cytotoxic molecules were released. It was a textbook case of rejection.
But the gene-edited cells? They survived. Not only did they go unnoticed by the immune system, but they also functioned as insulin-producing beta cells should.
C-peptide levels, a marker of insulin production, remained detectable and stable, even rising during mixed-meal tolerance tests. Glycated hemoglobin (HbA1c)—a measure of long-term blood sugar control—fell by approximately 42% over the course of the trial. PET scans using GLP-1 receptor-targeted tracers confirmed viable islet grafts with no signs of inflammation.
And perhaps most encouragingly, no serious side effects occurred. The patient continued his regular insulin therapy, as only a fraction of the needed beta-cell mass had been transplanted. But this trial was never meant to cure diabetes. It was meant to answer a question: Can gene-edited islets survive in a human without immune suppression?
The answer now appears to be yes.
What This Could Mean for Millions Living with Diabetes
This is not a cure—not yet. But it is a stunning proof-of-concept. For the first time, researchers have shown that human donor cells, modified with precision gene editing, can evade the immune system in a living person, function as insulin producers, and survive for months—all without drugs to suppress immunity.
If scaled and refined, this approach could radically change how we treat type 1 diabetes. Instead of managing the disease day by day, patients might one day receive curative cell therapy—long-lasting, self-regulating, and entirely drug-free.
Even for those with newly diagnosed disease, early implantation of hypoimmune beta cells might prevent the autoimmune attack altogether or buy precious years of normal pancreatic function.
More broadly, the implications extend beyond diabetes. If cells can be engineered to be invisible to the immune system, we might see breakthroughs in organ transplantation, cancer therapy, autoimmune disease, and even aging. The tools of gene editing are finally being paired with human trials—and the results are promising.
Caution, Questions, and the Road Ahead
Despite the excitement, much remains unknown. This trial involved only one patient, and for a relatively short period. Larger trials are needed to test long-term safety, efficacy, and durability. Could the immune system adapt over time and eventually recognize the edited cells? Could the genetic modifications lead to unintended consequences down the line? Could this approach be scaled for widespread use?
There’s also the question of how to produce enough cells. Right now, islet cells come from deceased donors—a scarce resource. But advances in stem cell-derived islets could eventually provide an unlimited supply of transplantable beta cells.
And what about cost? Gene editing, cell engineering, and transplantation remain expensive, complex, and logistically challenging. But as with many transformative technologies—from sequencing genomes to mRNA vaccines—costs can fall as knowledge and infrastructure grow.
What’s certain is that this trial marks a pivotal step. It represents the convergence of genetic engineering, regenerative medicine, and immunology in a single, elegant experiment—and it worked.
A Future Where the Body Accepts Help, Not Harm
For the man in the trial, it’s not yet a cure. He still takes insulin, still pricks his fingers, still counts carbohydrates. But for him—and for millions watching—this moment feels like something more than just another experiment.
It feels like hope made visible.
A future where beta-cell transplants work without wrecking the immune system. Where people with type 1 diabetes can live without the constant fear of lows, highs, complications, or side effects. Where the body accepts help—not as a foreign intruder, but as a long-lost ally returned.
It’s too early to celebrate. But not too early to dream.
Because for the first time in history, science has shown us that such a future may be possible.
More information: Per-Ola Carlsson et al, Survival of Transplanted Allogeneic Beta Cells with No Immunosuppression, New England Journal of Medicine (2025). DOI: 10.1056/NEJMoa2503822