Amid the quiet rustle of crops under the sun—where farmers once sowed seeds for food—scientists are now harvesting something far more extraordinary: a powerful tool in the fight against cancer.
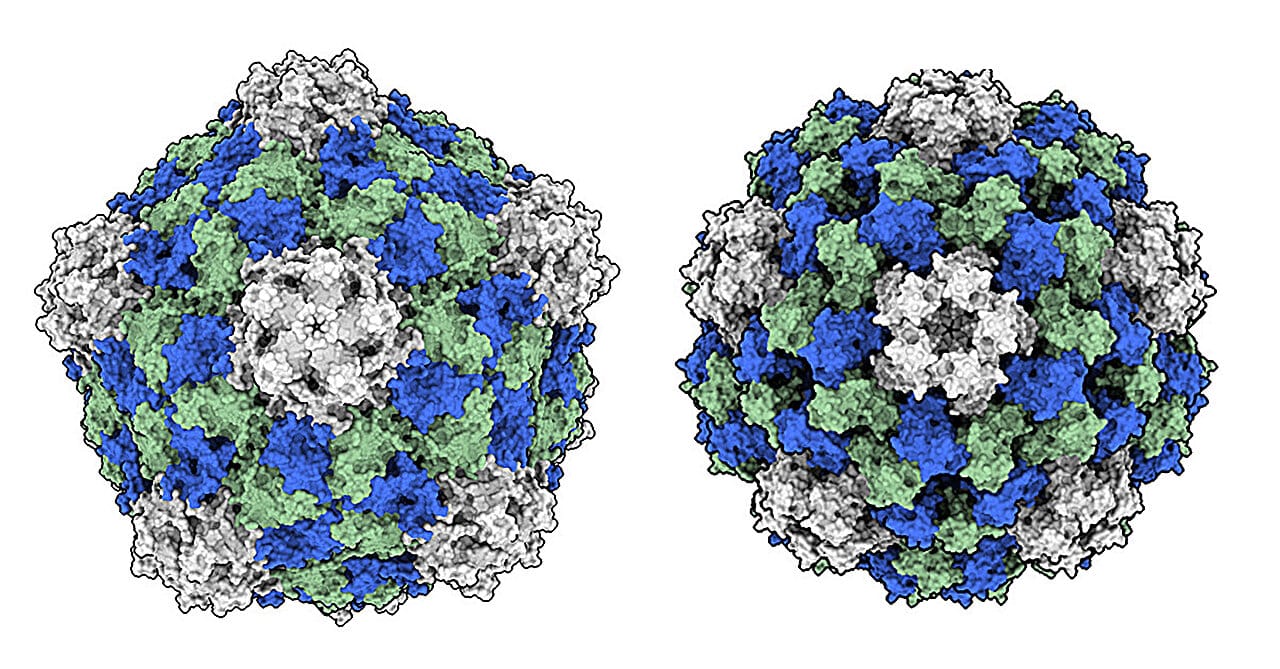
It may sound improbable, almost poetic, but the humble cowpea plant—known for producing the black-eyed pea—harbors a virus that could transform the way we treat one of humanity’s deadliest diseases. The virus is called cowpea mosaic virus (CPMV), and instead of causing harm, it may soon be helping humans survive the very thing that has defied medicine for centuries: metastatic cancer.
While many breakthroughs in immunotherapy have emerged from the complex world of synthetic drugs and gene editing, CPMV comes from nature’s own quiet library. And what makes this discovery so moving is not just its scientific brilliance, but its sheer simplicity: a plant virus that doesn’t even infect humans may succeed where some of our most expensive drugs have failed.
The Curious Case of a Plant Virus That Fights Cancer
The story begins not in a high-tech lab, but in the dirt—specifically, in the fields of cowpeas. CPMV is a plant virus that has long been studied for its unique structural properties. For decades, it was mostly of interest to botanists and virologists curious about how it infected legumes. But now, a team of researchers led by Dr. Nicole Steinmetz at the University of California San Diego has shown that this virus, when injected directly into tumors, can trigger the human immune system to do something remarkable: fight back.
In preclinical studies, including experiments on mouse models and even on pet dogs suffering from spontaneous cancers, CPMV has shown potent anti-tumor effects. The virus doesn’t target cancer cells directly like chemotherapy or radiation—it trains the immune system to do the job better. When introduced into a tumor, CPMV transforms the tumor microenvironment from a cold, silent battlefield into one roaring with immune activity.
Neutrophils, macrophages, natural killer cells—soldiers of the innate immune system—swarm the tumor site, triggered by the virus’s presence. And more importantly, T cells and B cells, which form the core of adaptive immunity, are awakened. They not only help eliminate the initial tumor but also remember it. If the cancer dares return or spread, the immune system is already waiting.
Not All Viruses Are Created Equal
But a lingering mystery remained: why CPMV? There are many plant viruses. Most are harmless to humans. Why is this one so uniquely effective at triggering such a profound anti-cancer response?
To answer this, Dr. Steinmetz’s team, along with collaborators at the National Cancer Institute’s Nanotechnology Characterization Laboratory, conducted a head-to-head comparison between CPMV and another closely related virus, cowpea chlorotic mottle virus (CCMV). Structurally, they are similar. Both are made up of nanoscale protein shells that encapsulate RNA. Both can enter human immune cells. And neither infects human tissue.
Yet only CPMV consistently results in tumor regression.
The reason, the team discovered, lies not in how the viruses look—but in how they behave once inside the body. CPMV uniquely triggers type I, II, and III interferons, a class of proteins essential to antiviral and anti-tumor immune responses. These are the same molecules once used as standalone drugs in early cancer immunotherapy. In contrast, CCMV activates only a set of interleukins that do not mount a meaningful attack against tumors.
Even more intriguing is how the viruses’ RNA is processed once inside mammalian immune cells. CPMV’s RNA reaches the endolysosome, a cellular compartment rich in Toll-like receptor 7 (TLR7)—a molecule that acts as an alarm bell for foreign invaders. Activation of TLR7 by CPMV RNA jumpstarts the immune system’s antiviral defenses. But instead of fighting a virus, these defenses turn their weapons on the tumor.
CCMV, meanwhile, fails to reach this critical threshold. Its RNA degrades before it can trigger the immune system’s full wrath. The difference, though microscopic, is monumental.
Immune Reprogramming Without Infection
What makes this phenomenon even more extraordinary is that CPMV does not infect human cells. It isn’t a pathogen. It doesn’t replicate in our tissues. Yet, immune cells respond to it as if it were a deadly threat.
“It’s fascinating,” said Anthony Omole, a Ph.D. student in Steinmetz’s lab and lead author of the study published in Cell Biomaterials. “Although human immune cells are not infected by CPMV, they are reprogrammed toward an activated state, which ultimately trains them to detect and eradicate cancerous cells.”
This reprogramming is a form of immune education—one that doesn’t rely on dangerous pathogens or weakened viruses like many traditional vaccines. It’s safe, elegant, and—remarkably—plant-based.
The fact that a simple plant virus can act as an immunological drill sergeant, commanding human cells to sharpen their defenses, opens the door to a radically different approach to immunotherapy—one that’s accessible, scalable, and surprisingly affordable.
A Therapy Grown by the Sun
Modern cancer therapies are notoriously expensive. CAR-T cell therapy, for instance, costs hundreds of thousands of dollars. Some checkpoint inhibitors are priced beyond the reach of many patients and healthcare systems. Even manufacturing these drugs involves high-tech facilities and strict biosafety protocols.
CPMV, in contrast, is refreshingly low-tech in origin. It can be grown in plants—in cowpea crops—with sunlight, soil, and water. This process, known as molecular farming, allows large quantities of CPMV to be produced in a cost-effective and environmentally friendly manner. It’s a therapeutic solution not grown in steel tanks or engineered with complex synthetic chemistry—but harvested from fields.
This affordability could dramatically democratize access to immunotherapy, particularly in low-resource settings or in countries where cutting-edge cancer care remains a distant dream. And because CPMV doesn’t pose any infection risk, it avoids many of the regulatory hurdles associated with viral therapies.
“We’re not just looking at a powerful immune stimulant,” Steinmetz explained. “We’re looking at one that can be scaled globally, without the cost barriers that come with most current immunotherapies.”
From Bench to Bedside
The work isn’t done. The UC San Diego team is now preparing to advance CPMV into clinical trials. Much remains to be tested, from optimal dosing to delivery methods, and from safety in human subjects to long-term efficacy. But the signs are promising. Decades of research in nanomedicine, immunology, and plant virology have all converged on this moment.
The virus that once caused harmless spots on cowpea leaves could one day give doctors a new weapon in the fight against cancer—a weapon that is effective, safe, and grown by the Earth itself.
“This is the time,” said Steinmetz. “We are poised to move this work beyond the bench and into the clinic. The data are solid. The mechanisms are understood. The path is clear.”
Hope Sprouting in Unexpected Places
In the end, the story of CPMV is a reminder that hope doesn’t always come from billion-dollar labs or genetically engineered cells. Sometimes, it springs from something ancient and overlooked. Sometimes, it’s been right in front of us the entire time—hiding in the roots of a legume, carried by a virus meant only for plants.
It is a testament to the ingenuity of nature—and to the curiosity of those who dared to look more closely.
What once grew only to nourish our bodies may now help save our lives. And that is not just science—it’s poetry written in proteins, told by the smallest storytellers on Earth.
Reference: Anthony O. Omole et al, Comparative analyses for plant virus-based cancer immunotherapy drug development, Cell Biomaterials (2025). DOI: 10.1016/j.celbio.2025.100095