In every cell of the human body, an invisible and tireless maintenance system works around the clock. Without this quiet labor, life would fall apart, molecule by molecule. This essential process is known as autophagy—a Greek term meaning “self-eating.” But don’t let the name alarm you. Autophagy is less about destruction and more about rejuvenation. It is the cell’s waste disposal and recycling program, disassembling worn-out or damaged components and transforming them into raw materials for renewal.
Without it, our cells would drown in their own clutter. Leftover proteins would clump and strangle cellular function. Mitochondria—the tiny powerhouses that fuel nearly every biological process—would become defective and toxic. Autophagy ensures that life can continue with clarity and order, even amid the entropy that threatens to pull it apart.
Among the many types of autophagy, one stands out for its connection to some of the most devastating neurological disorders of our time: mitophagy—the targeted degradation of mitochondria. When mitophagy fails, it is as if a city’s power stations go rogue. The energy system begins to poison its surroundings. And one of the diseases where this breakdown is most evident is Parkinson’s.
Now, a pioneering study out of the Max Perutz Labs at the University of Vienna has uncovered a new dimension to mitophagy—one that could redefine how we think about cellular cleansing and open doors to future therapies for Parkinson’s and other neurodegenerative conditions.
A Fresh Look at a Familiar Puzzle
For decades, the scientific consensus about mitophagy centered around a specific molecular circuit: the PINK1/Parkin signaling pathway. This well-characterized mechanism explained how cells recognized and removed defective mitochondria. PINK1, a protein kinase, accumulates on damaged mitochondria and recruits Parkin, a ubiquitin ligase, which marks the faulty organelles for autophagic destruction.
It was elegant, well-studied, and served as the model system for understanding mitophagy—until the scientists at the Max Perutz Labs decided to zoom out.
“When we looked at the big picture, it became clear that, apart from the much-studied PINK1/Parkin pathway, there were huge gaps in our knowledge of other mitophagy pathways,” explained Dr. Elias Adriaenssens, the study’s lead author.
Rather than retreading known ground, Adriaenssens and his colleagues ventured into the scientific equivalent of uncharted territory. What they found challenges core assumptions about how autophagy begins.
The Unexpected Role of WIPI Proteins
At the heart of this new discovery are two mitophagy receptors: NIX and BNIP3. These proteins had been known for years to participate in mitochondrial cleanup, especially during conditions like low oxygen or developmental stress. But their precise method of action remained elusive.
Traditionally, scientists believed that for mitophagy to be triggered, these receptors had to interact with a critical protein called FIP200. This protein was seen as an essential gatekeeper of autophagy—no FIP200, no cleanup.
But something didn’t add up.
In their experiments, the Vienna team conducted exhaustive tests and found no detectable interaction between FIP200 and either NIX or BNIP3. It was a dead end—or so it seemed.
Then came the surprise.
Using advanced mass spectrometry, the researchers identified unexpected partners in the process: WIPI proteins—a family of molecules previously thought to act later in the autophagy sequence. In a twist that rewrote the script, WIPI proteins were found binding directly to the mitochondrial receptors NIX and BNIP3.
This finding flipped the hierarchy of cellular waste management on its head.
Follow-up experiments confirmed the result. WIPI proteins, it appeared, weren’t just late-stage helpers—they were potential initiators of selective autophagy. In other words, cells may possess parallel routes to clean up their internal environment, depending on the stress, receptor, or context.
“This is an exciting discovery—it reveals a parallel trigger for selective autophagy,” said Adriaenssens. “Until now, no one has considered WIPI proteins to be key players in triggering autophagosome formation, but our discovery could change that view.”
Multiple Roads to the Same Destination
What this means is that cells might use more than one route to clear damaged mitochondria. The PINK1/Parkin pathway may not be the sole guardian of mitophagy. Instead, different receptors like NIX and BNIP3 can act independently, triggering cleanup via alternative molecular messengers—particularly the WIPI proteins.
This isn’t just an academic curiosity. It raises profound questions about how cells decide which route to take. Is it a matter of timing? Of stress intensity? Of the kind of damage involved?
The researchers believe that cells might choose different mitophagy pathways based on their current condition. And if one pathway is broken—due to genetic mutations or disease—perhaps another could be intentionally activated to compensate.
This possibility opens a tantalizing therapeutic window.
In Parkinson’s disease, for example, the PINK1/Parkin pathway is often disrupted. If scientists could enhance the WIPI-dependent pathway in patients, they might bypass the faulty system altogether, restoring mitochondrial health and potentially slowing or even halting the progression of neurodegeneration.
Why Parkinson’s Begins in the Cell’s Power Grid
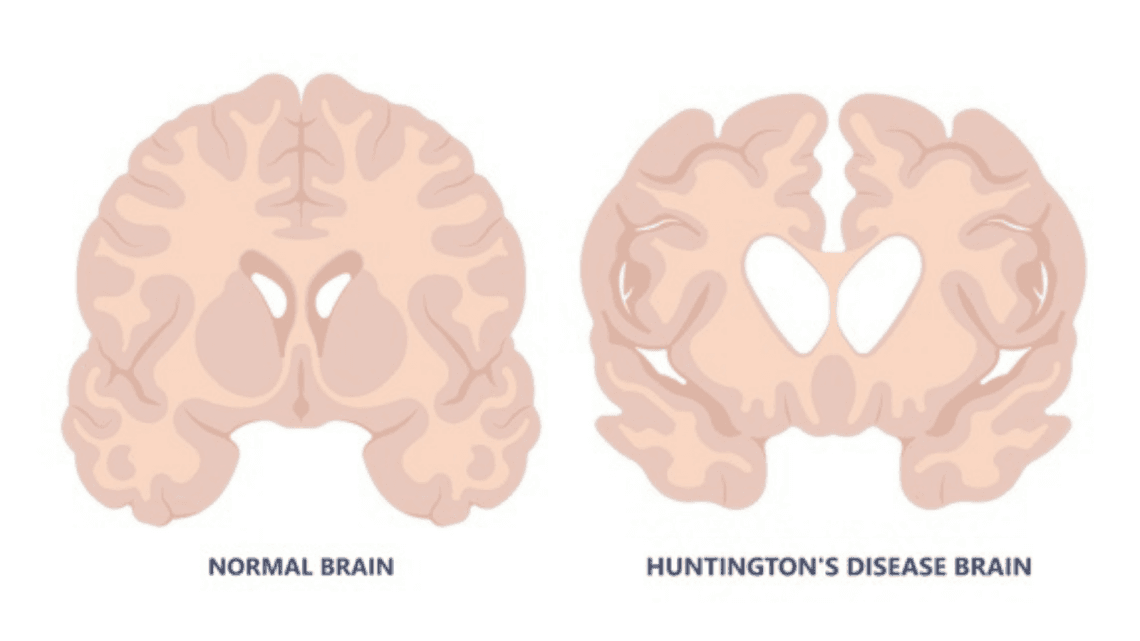
Parkinson’s disease is one of the most well-known neurodegenerative disorders, marked by the slow death of dopamine-producing neurons in the brain. But long before tremors and stiffness appear, a deeper molecular tragedy is unfolding inside those neurons.
Mitochondria begin to fail.
They stop producing energy efficiently. They leak damaging molecules. They signal distress. Normally, mitophagy would sweep in, identifying and recycling these defective mitochondria. But in many Parkinson’s patients, this process is stunted—either because of mutations in the genes that code for PINK1 and Parkin or due to failures in the machinery that follows them.
If WIPI proteins offer a backup route to initiate cleanup, they may hold the key to early, effective interventions. By boosting the activity of NIX and BNIP3 or by enhancing the WIPI-dependent recruitment of autophagy machinery, scientists could develop treatments that help neurons stay healthy longer.
Rethinking Cellular Order
Perhaps the most remarkable aspect of this study is not just its findings, but its implications for how we think about biological systems. For years, autophagy was treated as a linear pathway—a one-way street from detection to degradation. This research shows that, in reality, the system is more flexible, dynamic, and redundant than previously imagined.
Cells don’t rely on a single method to manage their waste. They adapt, they improvise, they evolve new routes when old ones fail. That resilience may be their greatest strength—and our greatest hope in designing new treatments.
As Adriaenssens notes, “Instead of a single, universal mechanism, cells appear to use different molecular strategies depending on the receptor and context.”
It’s a bit like having multiple emergency exits in a building. If the main door is blocked, another route may save you. This redundancy, once viewed as noise in the system, now looks like a blueprint for survival.
A New Frontier for Therapies
The path from fundamental cell biology to clinical application is long, often taking decades. But discoveries like this one lay the essential groundwork. With the ability to manipulate WIPI pathways, researchers could one day design small molecules, peptides, or gene therapies that reprogram mitophagy from within.
Such treatments wouldn’t just be useful for Parkinson’s—they could benefit patients with Alzheimer’s, ALS, and other conditions where cellular waste accumulates unchecked. Even certain cancers, which manipulate autophagy for survival, could become vulnerable to therapies that redirect these newly discovered pathways.
And in the meantime, the discovery offers something else: a sense of momentum, a new direction for a field that has for too long been dominated by a single pathway.
The Quiet Revolution Inside Every Cell
What this study from the Max Perutz Labs reminds us is that even in the smallest spaces of biology—in the dark, crowded interior of a human cell—there is mystery, drama, and discovery. Molecules whisper to one another across membranes. Proteins form fleeting alliances. Signals cascade like falling dominoes. And occasionally, someone listens carefully enough to hear a new story.
Autophagy may be the cell’s rubbish collection system, but it is also a mirror of life’s deeper truths: that survival often depends on what we let go of, what we recycle, and how we adapt to the things that break.
In discovering a new way that cells take out the trash, scientists have not only deepened our understanding of disease—they’ve renewed our hope in the body’s quiet, persistent wisdom.