Imagine if cancer could be detected early—before it spreads, before symptoms appear, before it begins to chip away at the chances of survival. This isn’t just a hopeful dream; it’s the ultimate challenge in oncology. Most cancers, especially lethal ones like pancreatic, ovarian, and liver cancer, are typically diagnosed too late, after the disease has already made its silent march deep into the body. Traditional screening tools, while life-saving for a select few cancers like breast and colon, leave vast gaps. Many cancers don’t have any screening tests at all. But what if there were a single, non-invasive test that could spot multiple cancers at once, pinpoint where they originated, and do so with high accuracy?
In a groundbreaking study published in Nature Medicine, researchers from Geneseeq and a collaborative network of Chinese academic hospitals have taken a massive step toward that vision. Their innovation—a multi-cancer early detection (MCED) blood test—relies on a relatively new and rapidly evolving biomarker: cell-free DNA (cfDNA). With astonishing sensitivity and specificity, this test doesn’t just hint at the presence of cancer. It illuminates it, drawing a molecular map of where the rogue cells may be hiding.
Decoding Molecular Whispers in the Bloodstream
Cancer, even in its earliest stages, leaves a molecular fingerprint in the body. As tumor cells grow and die, they release fragments of their DNA into the bloodstream. These small pieces, known as cell-free DNA, float silently through the plasma, offering a noninvasive window into the body’s inner workings. In healthy individuals, cfDNA primarily originates from normal cells. But in cancer patients, a portion of this DNA—called circulating tumor DNA (ctDNA)—derives from malignant cells.
Unlike the mutated genes commonly targeted in tumor biopsies, cfDNA holds secrets in its very structure. Fragment size, patterns of degradation, chromatin accessibility, and methylation marks can all reveal whether DNA comes from a healthy cell or a cancerous one. This field of analysis is called fragmentomics, and it’s rapidly becoming a powerful new language in cancer diagnostics.
What Geneseeq and its partners have done is leverage whole-genome sequencing and machine learning to interpret these molecular whispers. Their test doesn’t look for one or two known mutations. It listens to the entire symphony of cfDNA signals, extracting patterns that can distinguish cancerous from noncancerous samples and even predict the tumor’s organ of origin.
An Algorithm Trained on Thousands of Genomes
To train this molecular detective, researchers assembled one of the largest datasets of its kind. They collected blood samples from 3,076 cancer patients spanning over a dozen tumor types and matched them against 3,477 non-cancer controls. These samples were processed under a rigorous double-blind protocol, ensuring that neither the lab technicians nor the data analysts knew the clinical status of each participant.
The researchers used low-coverage whole-genome sequencing, a cost-effective approach that captures broad patterns across the genome rather than zooming in on a handful of known mutations. This technique allows for the detection of changes in copy number variations, nucleosome positioning, fragment length distributions, and even inferred methylation states—all of which carry the hallmarks of cancer biology.
From this data, they trained two supervised machine learning classifiers. One model was optimized to detect whether a cancer signal was present; the other was built to guess where that signal was coming from. Importantly, the models were frozen before validation began. This means that no further tweaking or optimization was allowed once they started testing on new data, reducing the risk of overfitting and increasing the real-world reliability of their performance.
The Numbers That Could Save Millions
When tested on an independent validation cohort of 1,465 participants, the MCED test delivered results that surpassed expectations. It correctly identified the presence of cancer with 87.4% sensitivity and 97.8% specificity. These two metrics are critical: sensitivity tells us how good the test is at finding actual cancer cases, while specificity tells us how good it is at avoiding false alarms in healthy individuals. For a test of this kind, balancing these two has always been the Achilles’ heel. High sensitivity often means too many false positives; high specificity usually comes at the cost of missing early cancers. Here, researchers struck a remarkable balance.
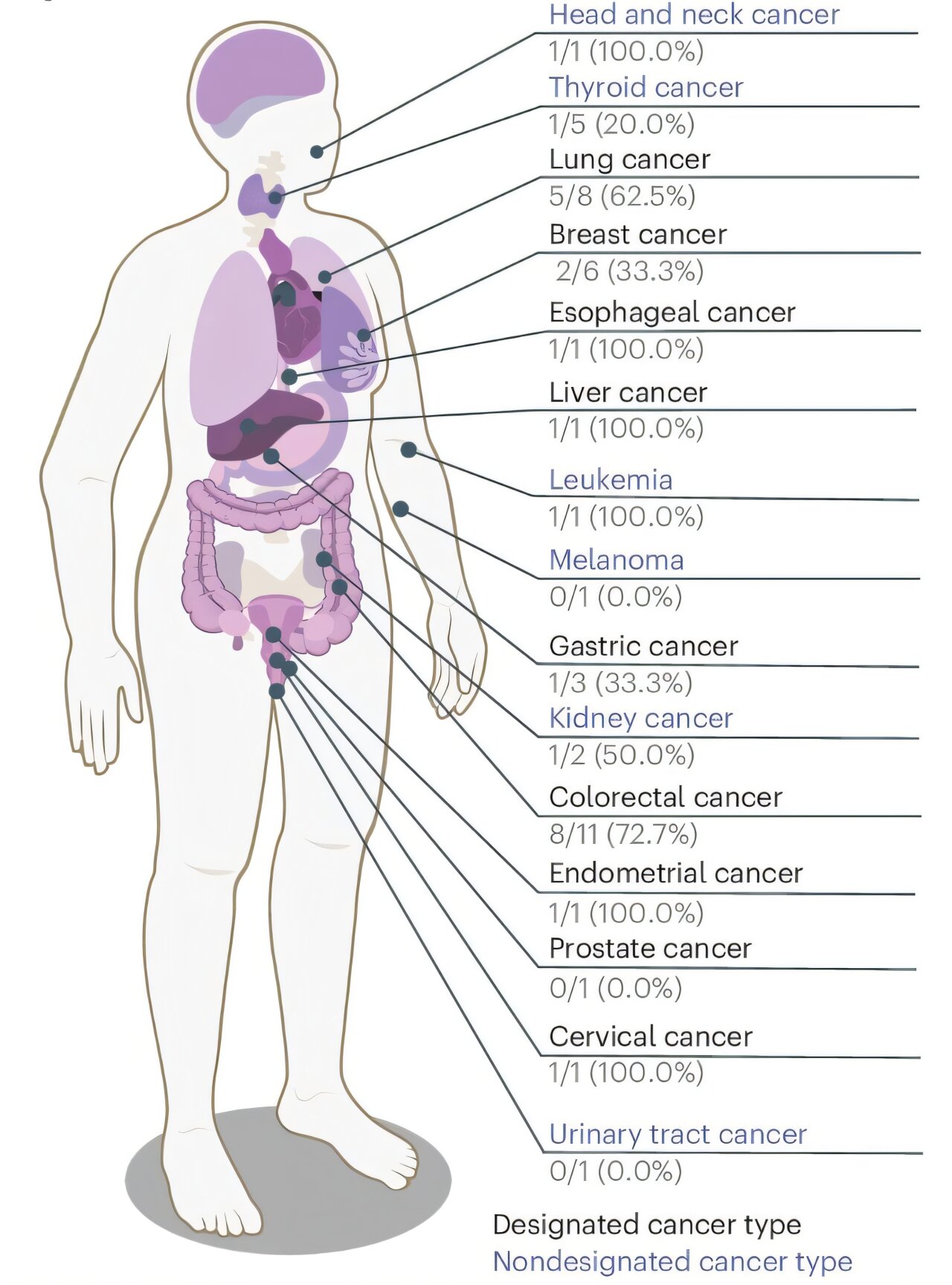
Even more impressive was the test’s ability to identify early-stage disease. For stage I cancers, sensitivity was 79.3%, and for stage II, it rose to 86.9%. These stages represent the critical window for intervention, where treatments are most effective and survival rates are significantly higher. For notoriously elusive cancers like pancreatic cancer, the test detected 76.9% of cases overall and even 58.3% of stage I cases—an extraordinary achievement considering the current lack of screening options.
For liver and bile duct cancers, sensitivity reached a perfect 100%, and for lung, ovarian, and colorectal cancers, it exceeded 80%. The test was not only catching cancers—it was catching the deadliest kinds in their infancy.
A Compass for Cancer’s Origins
But detecting cancer is only part of the story. Once a test flags a positive, clinicians need to know where to look. That’s where tissue-of-origin prediction becomes indispensable. Using the same cfDNA patterns, the machine learning model also predicted the likely origin of the tumor. In the independent validation group, the test got it right 83.5% of the time based on the top-ranked guess, and 91.7% when considering the top two most likely locations. That level of accuracy could help doctors narrow down diagnostic imaging or guide biopsies, turning a molecular signal into a clinically actionable plan.
Real-World Testing in Healthy Populations
To truly understand the test’s potential in the real world, the researchers didn’t stop at retrospective data. They launched a prospective study called the Jinling cohort, enrolling 3,724 asymptomatic individuals across two Chinese medical centers. Participants were followed for one year to see which ones developed cancer.
During that time, 43 cases of cancer emerged. The MCED test identified 53.5% of these cases early—before they were picked up by traditional means—and maintained an outstanding 98.1% specificity. Importantly, nearly half of the detected cancers were missed by standard screening methods or physical exams. The test’s positive predictive value was 25%, and its negative predictive value was a comforting 99.4%, suggesting that a negative result truly means low risk.
Tissue-of-origin accuracy in this real-world group was a bit lower than in the controlled validation set—63.2% for the top guess and 89.5% for the top two—but still remarkably useful in the clinical context.
The Promise and the Path Ahead
This study doesn’t just validate a test. It signals a paradigm shift in how we think about cancer detection. Traditional methods are like flashlights in the dark, lighting up one narrow beam—mammograms for breast, colonoscopies for colon, and little else. This new test is more like a searchlight, sweeping broadly across the body for dozens of cancer types at once, and shining brightest on those that are usually hidden.
The implications are profound. With further refinement and large-scale deployment, this type of MCED test could become a routine part of annual health checkups. It could identify cancers long before symptoms arise, guiding early intervention, reducing treatment costs, and most importantly, saving lives. And because it’s based on a simple blood draw, it’s accessible in a way that invasive biopsies or complex imaging scans are not—particularly in regions with limited healthcare infrastructure.
Yet, challenges remain. While specificity is high, the modest positive predictive value in asymptomatic individuals underscores the need for confirmatory diagnostics to avoid unnecessary anxiety or procedures. Cost, regulatory approval, and integration into healthcare systems are also hurdles. But the momentum is building, and the technology is maturing.
A New Frontier in the War on Cancer
This is not just a story about a diagnostic tool. It’s a story about how science, when combined with technology and imagination, can reveal what once seemed invisible. The ability to see cancer forming—not with a scalpel or a scope, but with data—represents a seismic leap in medicine.
The Geneseeq team and their collaborators have brought us closer than ever to catching cancer in the act. Their work transforms cfDNA from a cryptic molecular fragment into a powerful signal, deciphered by the twin engines of genomics and machine learning. And with that, the once-impossible dream of early, pan-cancer detection inches closer to reality.
In their own words, the researchers conclude that their findings “… indicate that the MCED test has strong potential to improve early cancer detection and support clinical decision-making.” But even that feels like an understatement. What they’ve demonstrated is the beginning of a future where catching cancer early is not an accident, but an expectation.
The fight against cancer has long been reactive. This test—and the science behind it—ushers in a new era of proactive, predictive, and personalized care, where disease may one day be stopped not in the operating room, but with a drop of blood and a whisper of fragmented DNA.
Reference: Hua Bao et al, Early detection of multiple cancer types using multidimensional cell-free DNA fragmentomics, Nature Medicine (2025). DOI: 10.1038/s41591-025-03735-2