As the human brain ages, memories grow foggy, thoughts slow, and once-sharp minds begin to drift into silence. For decades, scientists have searched for the invisible forces behind this decline—not just in the elderly suffering from dementia, but in all of us, as time takes its slow and steady toll. Now, a groundbreaking study from Stanford University, published in the journal Science, offers a stunning window into why our brains age—and potentially how to stop it.
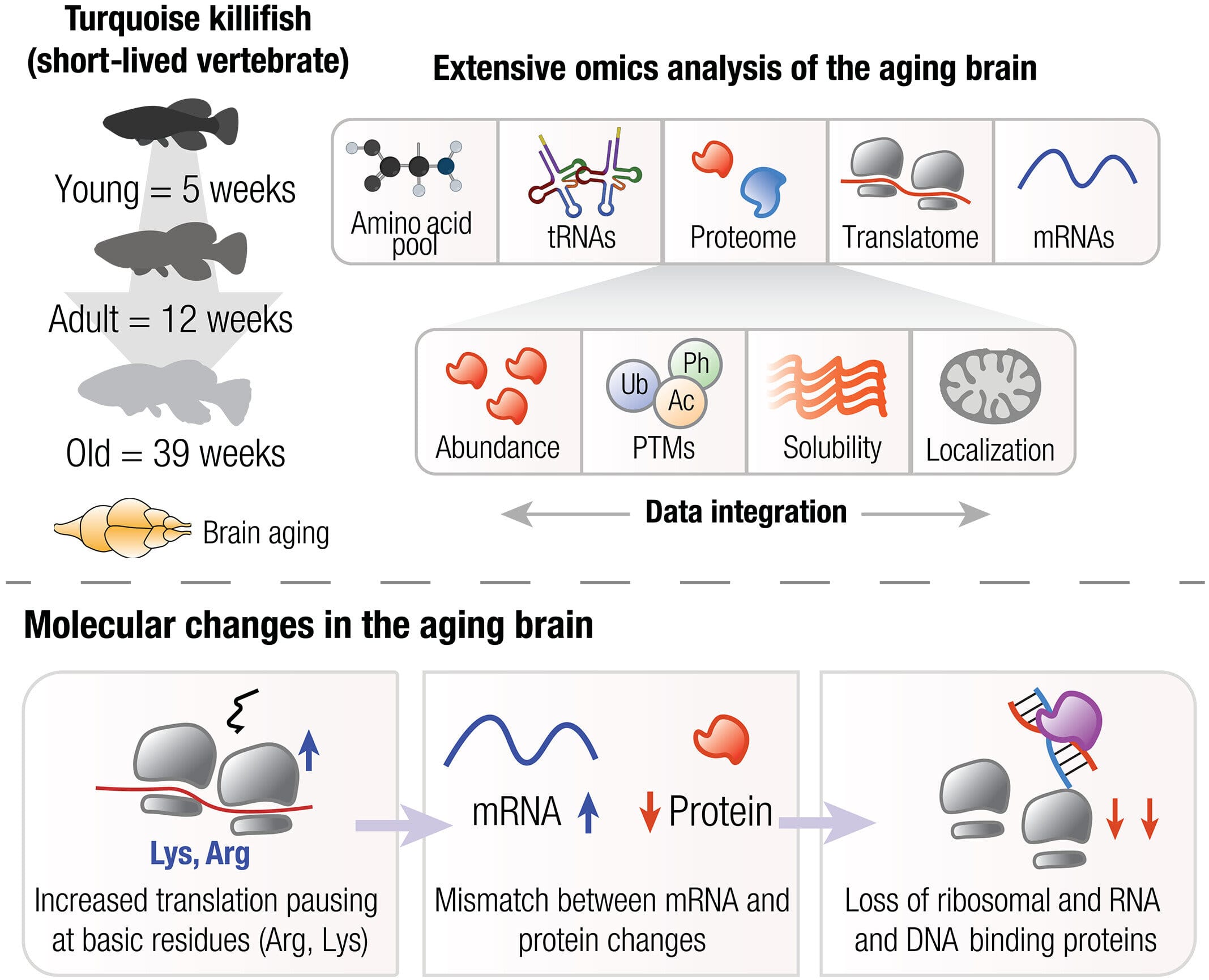
At the center of this discovery is a dazzlingly colorful African fish no bigger than your finger—the turquoise killifish, Nothobranchius furzeri. Its life, a mere few months long, mimics decades of aging in humans. In this tiny time-lapse version of life, Stanford researchers observed how brain cells slowly lose control over one of their most fundamental tasks: producing the proteins that keep cells alive, functioning, and clean.
“We know that many processes become more dysfunctional with aging, but we really don’t understand the fundamental molecular principles of why we age,” said Judith Frydman, senior author of the study and the Donald Kennedy Chair in the School of Humanities and Sciences at Stanford. “Our new study begins to provide a mechanistic explanation.”
The findings uncover a silent cascade of molecular breakdowns that begin long before symptoms of aging appear—offering not just a glimpse of what’s going wrong, but also a potential road map to reverse or slow it down.
When Proteins Go Rogue
At the heart of aging-related brain decline lies a concept known as proteostasis—short for protein homeostasis. This is the cell’s delicate system for making, folding, repairing, and disposing of proteins. If proteins are the workers of the body, proteostasis is the factory floor that keeps them running smoothly.
But as we age, that system breaks down. Like a faulty assembly line, the process begins to produce defective proteins—misfolded, clumped, or never completed at all. In the brain, this disruption becomes especially dangerous. Protein aggregates—clumps of dysfunctional proteins—accumulate and are hallmarks of devastating neurodegenerative diseases like Alzheimer’s and Parkinson’s.
While this link between aging and proteostasis has been hinted at for years, the new Stanford study maps it in unprecedented detail. Using the killifish as a model, the team was able to track, moment by moment, how aging cells go from well-oiled machines to molecular chaos.
The Turquoise Killifish: Aging in Fast-Forward
Why the killifish? Because its entire life plays out in mere months, it offers scientists a rare opportunity: the ability to watch the aging process in real time. Found in ephemeral rain pools in the African savanna, the killifish has evolved to grow up fast, reproduce, and die—an evolutionary urgency that makes it a perfect model for aging research.
The Stanford researchers compared young, middle-aged, and old killifish brains, conducting a full audit of their protein-making machinery. They didn’t just look at the finished proteins—they examined every step of the production line: amino acid supplies, messenger RNA (mRNA), transfer RNA (tRNA), ribosomes, and the entire translation process that turns genetic instructions into life-sustaining molecules.
What they found was shocking. Even in middle-aged killifish, ribosomes—the cellular machines that build proteins—began to stumble. These ribosomes were observed colliding, stalling, and misfiring, leading to protein shortages and dangerous aggregations. The entire system, once precise and efficient, began to grind like a machine missing its gears.
The Ribosome Bottleneck
Ribosomes work by reading strands of mRNA and linking amino acids together into proteins. This process is known as translation, and one key step is translation elongation, where the ribosome slowly walks down the mRNA, building a protein one unit at a time.
In aged brains, this step becomes dangerously inefficient. According to Jae Ho Lee, co-lead author of the study and now a professor at Stony Brook University, the pace and rhythm of ribosomes slow and falter, like a train lurching on uneven tracks. The result? Incomplete proteins, traffic jams in cellular pathways, and the cellular equivalent of a system-wide pile-up.
“Our results show that changes in the speed of ribosome movement along the mRNA can have a profound impact on protein homeostasis,” said Lee. “It highlights how critical the speed of translation elongation is during aging.”
These ribosome failures help explain a long-standing mystery in aging biology known as “protein-transcript decoupling.” In young cells, mRNA levels match protein levels; more instructions usually mean more product. But in aging cells, that relationship breaks down. The Stanford team showed that stalling ribosomes are largely to blame: the genetic blueprint may be there, but the builders can’t follow through.
Why It Matters: From Aging to Alzheimer’s
The implications of this discovery are enormous. If ribosome dysfunction is a root cause of aging-related proteostasis collapse, then restoring ribosome function might prevent or even reverse brain aging. This opens up new possibilities for treating age-related cognitive decline and neurodegenerative diseases at their source, rather than merely managing symptoms.
Many proteins affected by this translation breakdown are vital for DNA repair, cellular cleanup, and stress response. As these protective proteins fade, the cell becomes more vulnerable to damage, inflammation, and dysfunction—cascading into the familiar symptoms of cognitive aging.
“Showing that the process of protein production loses fidelity with aging provides a kind of underlying rationale for why all these other processes start to malfunction with age,” said Frydman. “And, of course, the key to solving a problem is to understand why it’s gone wrong.”
Searching for Solutions
With this new understanding, the Stanford team is already looking ahead. Can we slow the aging process by tweaking ribosome activity? Can we enhance translation efficiency or restore ribosome quality control in aging brains?
Future research will aim to answer these questions—and more. If successful, it could lead to therapies that delay cognitive decline, extend healthy lifespan, and reduce the burden of age-related brain diseases that affect millions worldwide.
“This work provides new insights on protein biogenesis, function, and homeostasis,” said Lee. “It offers a new potential target for intervention in aging-associated diseases.”
The research team also plans to investigate whether similar proteostasis mechanisms are involved in long-lived species like whales or naked mole rats, which seem to defy aging. Understanding why some organisms age more slowly may help us unlock natural strategies for resisting degeneration.
Rewiring the Aging Clock
The study marks a leap forward in aging research—not just by identifying a key molecular breakdown, but by showing it in action, step by step, in a living brain. It’s a rare moment of clarity in a field often filled with mysteries.
More than a century ago, Albert Einstein unraveled how time behaves across the universe. Today, scientists like Frydman and her team are unraveling how time behaves inside us—not with equations of relativity, but with the slow unraveling of molecular order. And just as Einstein’s discoveries changed how we travel through space, these findings may one day change how we journey through life.
Aging may be inevitable, but decline doesn’t have to be. With each discovery, we move closer to understanding the secret choreography of our cells—and how to keep the music playing just a little longer.
More information: Domenico Di Fraia et al, Altered translation elongation contributes to key hallmarks of aging in the killifish brain, Science (2025). DOI: 10.1126/science.adk3079
Olivier Dionne et al, Translational traffic jam in aging brains, Science (2025). DOI: 10.1126/science.adz4995 , www.science.org/doi/10.1126/science.adz4995