For years, doctors and patients alike have witnessed the benefits of exercise in easing the tremors and stiffness of Parkinson’s disease. But the mechanisms behind these benefits have long remained a mystery. Now, a team of researchers in Cleveland may have brought us one step closer to understanding how exercise changes the brain—possibly for good.
In a compelling new pilot study published in Clinical Neurophysiology, scientists at University Hospitals and the VA Northeast Ohio Healthcare System, through the Cleveland Functional Electrical Stimulation (FES) Center, have shown that long-term dynamic cycling exercise might do more than temporarily relieve symptoms. It may actually help restore brain circuits damaged by Parkinson’s disease.
At the heart of the study lies an unusual and exciting combination: high-tech stationary bikes, deep brain stimulation (DBS) implants, and a vision of healing the brain through motion.
How Movement Became Medicine
Parkinson’s disease is a progressive neurological disorder that affects movement, causing tremors, stiffness, and difficulty with balance and coordination. These motor symptoms are caused by the loss of dopamine-producing neurons in a part of the brain known as the basal ganglia. Deep brain stimulation, or DBS, is a well-established treatment that delivers electrical impulses directly to this region, helping control symptoms when medication alone is no longer effective.
But even with DBS, Parkinson’s continues to march forward. That’s why the new study—led by Dr. Aasef Shaikh, neurologist and professor at University Hospitals and the Cleveland VA Medical Center—focused on something far more ambitious than symptom relief. What if, researchers wondered, exercise could actually retrain the brain’s motor circuits?
“Over years of study, we’ve already seen that dynamic cycling helps relieve tremor,” Dr. Shaikh said. “Now, we’re asking a deeper question: How? What’s changing in the brain?”
A Team Effort and a Veteran-Focused Vision
The study brought together a powerful collaboration between academic medicine and veterans’ health care. Participants were recruited from both University Hospitals and the Louis Stokes Cleveland VA Medical Center, including military veterans already living with Parkinson’s.
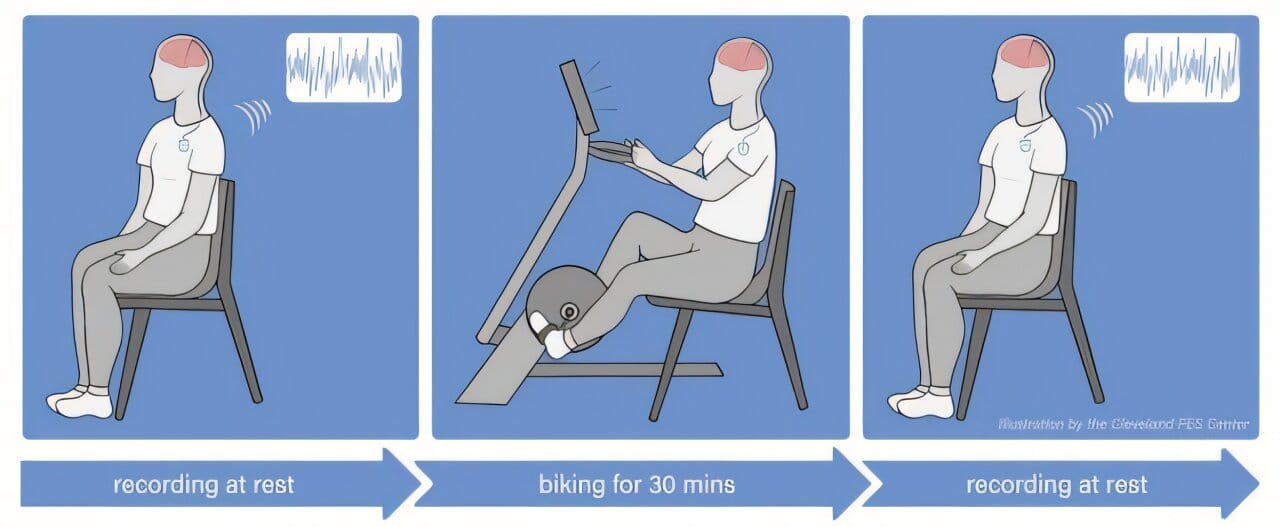
Every participant had previously been implanted with a second-generation deep brain stimulation device, capable not only of delivering therapy but also of recording the electrical activity inside the brain. This feature allowed researchers to monitor, in real time, how exercise affected the brain’s motor control regions—particularly the subthalamic nucleus, a common DBS target.
This kind of direct insight into the human brain is incredibly rare. It provided the team a chance to observe brain activity before and after every exercise session, painting a detailed picture of what might be changing beneath the surface.
The Bike That Learns With You
But this wasn’t just any exercise program. Participants took part in 12 adaptive cycling sessions over four weeks, using specially designed stationary bikes that don’t just follow commands—they respond to the rider.
The technology behind these bikes is both simple and subtle. Riders view a game screen while pedaling, instructed to maintain a cadence of 80 revolutions per minute. Their effort is visualized as a balloon, which they must keep afloat on screen—above water, but below a ceiling. The catch? The bike doesn’t just offer a fixed resistance. It adapts in real time, challenging each rider by increasing or decreasing the resistance based on their unique performance.
Kent State Ph.D. candidate Lara Shigo, a co-author of the study, says this method trains the brain and body in ways that traditional exercise simply doesn’t. “Eighty RPMs is faster than most people would naturally choose to pedal,” she explains, “but because the motor helps, riders aren’t exhausted. They’re engaged. They’re learning.”
That element of surprise—the “push and pull” of motor-assisted effort—may be key to how this kind of exercise stimulates the brain.
Rewiring the Network
Lead author Prajakta Joshi, a Ph.D. candidate in biomedical engineering at the Shaikh Lab, helped analyze the trove of neural data collected from the participants’ DBS electrodes. Their goal: determine whether the exercise sessions caused any measurable change in the brain’s electrical activity.
The answer wasn’t immediate. Early in the program, brain signals remained largely unchanged. But by the end of the 12 sessions, researchers detected a shift.
“We saw a measurable change in the brain signals associated with motor control,” said Dr. Shaikh. These changes weren’t just flickers of activity—they were signs that deeper circuits might be reorganizing.
Joshi emphasized that while current DBS systems can only record from the areas where electrodes are implanted, the ripple effects could extend far beyond. “There may be a broader circuit involved,” she explained. “We believe upstream and downstream pathways could also be influenced. What we’re seeing might be a network-level change—one that rewires the brain, not just in one spot, but across the whole motor system.”
From Symptoms to Systems
The implications of this are profound. Parkinson’s disease has long been treated as a localized condition—a loss of cells in one small brain region. But newer research is beginning to view it as a system-wide disorder, with symptoms arising from breakdowns in communication between many parts of the brain.
This study supports that newer view. It suggests that dynamic exercise doesn’t just strengthen muscles or boost dopamine—it may help the brain reconnect with itself.
Dr. Shaikh points out that the brain’s plasticity—its ability to adapt and reorganize—is likely being leveraged by the consistent, targeted stimulation of adaptive exercise. “We’re not just seeing improvements on the outside,” he said. “We’re watching the brain learn again.”
The Path Ahead
Though the study was small, its results are galvanizing. The next phase of research will expand both the number of participants and the technologies used, potentially incorporating brain imaging and more advanced DBS devices that can capture signals from a wider range of regions.
Joshi and her team are also interested in exploring whether other forms of physical activity—dancing, boxing, or yoga—could offer similar neural benefits when paired with real-time monitoring.
The goal, they say, is not just better symptom relief, but personalized neurological therapy. A future where exercise regimens could be tailored based on brain feedback, where doctors might prescribe a cycling program as precisely as a pill.
“We’re on the edge of something big,” Joshi said. “This is not just about slowing Parkinson’s—it’s about teaching the brain how to move again.”
The Human Story Behind the Science
What makes this study so compelling isn’t just the data—it’s the humanity woven through it. The participants, many of them veterans, didn’t just ride bikes. They fought to reclaim control over their own bodies. They trained through fatigue, braved the uncertainty of research, and placed their hope in a future not yet written.
Each pedal stroke was an act of defiance against the disease that tries to silence movement. And each neural signal recorded was a reminder that the brain, even when wounded, still holds the power to heal.
Science often advances in quiet revolutions. This one hums to the rhythm of pedals spinning—80 revolutions per minute, toward a new frontier in Parkinson’s care.
More information: Prajakta Joshi et al, Electrophysiological correlates of dynamic cycling in Parkinson’s disease, Clinical Neurophysiology (2025). DOI: 10.1016/j.clinph.2025.03.018