In the high-stakes world of neuroscience and genetic medicine, scientists have long searched for reliable ways to deliver genetic material into the human brain without disturbing its intricate circuitry or triggering devastating side effects. Adeno-associated virus (AAV) vectors have emerged as molecular couriers of extraordinary potential—tiny, reengineered viruses capable of ferrying custom-designed DNA directly into cells.
Recent breakthroughs from the Armamentarium consortium—a collaborative initiative involving some of the world’s most innovative minds in neuroscience, virology, and genetic engineering—have taken this potential one giant leap further. By tailoring these AAV tools with unprecedented precision, scientists are now targeting some of the most elusive cell types in the human brain.
At the helm of two new studies advancing these capabilities is Xiangmin Xu, Ph.D., Chancellor’s Professor of Anatomy and Neurobiology at the University of California, Irvine, and director of the Center for Neural Circuit Mapping. His team, along with collaborators from UC San Diego and the Allen Institute for Brain Science, has developed highly specific AAV-based vectors designed to overcome the infamous barriers that have historically stymied brain-targeted drug delivery.
“This Armamentarium’s collection of work enables new tools that help to deepen our understanding of the human central nervous system’s structure and function,” says Xu. “Our own brain-targeting technology could help treat Alzheimer’s disease and many other neurological disorders.”
Their latest findings, published simultaneously in the high-impact journals Neuron and Cell Reports Methods, detail two cutting-edge systems for targeting brain endothelial cells and excitatory neurons, respectively—two cell populations critical to brain health, yet historically difficult to reach. Together, these studies are not just technical marvels—they could pave the way toward life-saving therapies for neurodegenerative diseases, stroke, and even disorders of cognition and memory.
The Blood-Brain Barrier: An Impenetrable Fortress
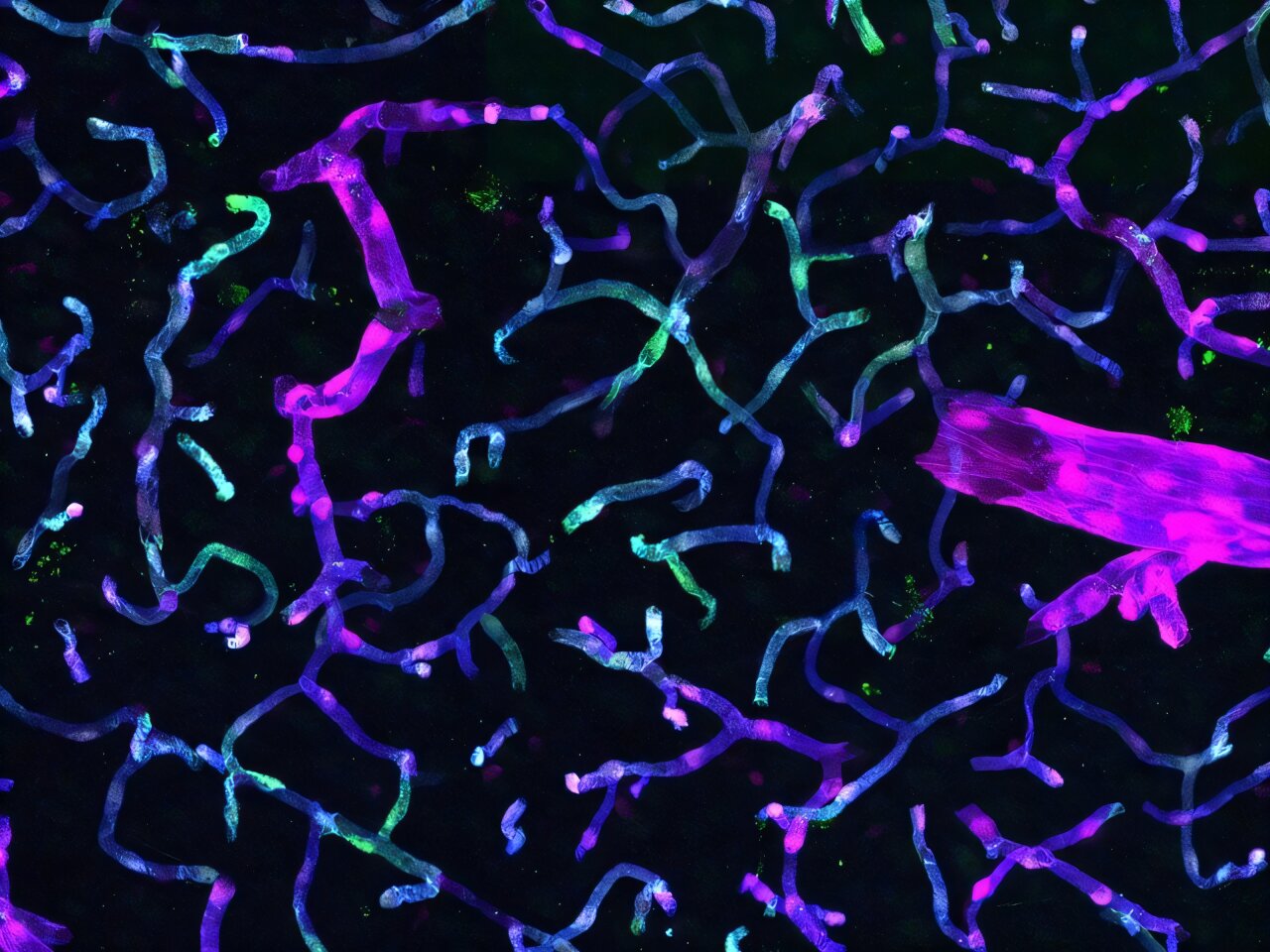
The human brain is protected by a remarkable structure called the blood-brain barrier (BBB), a tightly regulated border of specialized endothelial cells lining the cerebral blood vessels. This barrier allows the brain to keep harmful pathogens and toxins at bay, maintaining a pristine environment for neural activity. But the very mechanisms that keep the brain safe also make it maddeningly inaccessible to most drugs and gene therapies.
“Drug delivery to the brain is a big challenge due to this barrier,” explains Eric Velazquez-Rivera, a postdoctoral researcher in the Xu Lab and lead author of one of the new studies. “But we have developed a way to circumvent the BBB with a technology that targets brain vasculature with minimal unwanted side effects.”
That technology involves engineering AAV vectors equipped with genomic enhancer sequences—short pieces of DNA that dramatically boost the expression of therapeutic genes, but only in specific cell types. In this case, the targets are brain endothelial cells (BECs), the very cells that make up the BBB itself.
The researchers’ new vector, described in their Neuron article titled “Specific targeting of brain endothelial cells using enhancer-AAV vectors,” was built with an intricate understanding of the unique features that differentiate brain blood vessels from those in the rest of the body. The result? A highly specific AAV vector that can deliver genes directly to BECs—without accidentally affecting other tissues.
Precision Strikes in Alzheimer’s Models
What’s especially exciting is how well this BEC-targeting vector performs in disease contexts. The team tested their new AAV tool in a mouse model of Alzheimer’s disease known for its aggressive pathology. Even amid the neuroinflammatory chaos of Alzheimer’s, the vector maintained its targeting precision, focusing solely on brain endothelial cells and avoiding off-target expression.
“The capability to target with high specificity the endothelial cells in the brain can open the door to the development of new gene therapy vectors for a broad array of neurological diseases,” says Velazquez-Rivera.
The implications are sweeping. For instance, many neurodegenerative diseases—Alzheimer’s, Parkinson’s, Huntington’s—are thought to involve vascular dysfunction and disrupted blood-brain barrier integrity. By targeting the very cells that mediate these barriers, gene therapy could now directly restore or modulate their function.
“Our BEC AAV vector tools could be used for brain-targeted gene therapy to ameliorate neurological diseases,” Velazquez-Rivera adds. “It may even be used someday to treat stroke victims.”
Stroke, after all, is not just a matter of blood clots or hemorrhages—it is a devastating event that disrupts the blood-brain interface and triggers cascades of neuronal death. The potential to repair this interface quickly and precisely could mean the difference between permanent disability and full recovery.
A Vision Realized: Engineering Hope
According to Todd Holmes, Ph.D., professor of physiology and biophysics at UC Irvine and senior author of the study, the seeds for these breakthroughs were planted years ago.
“Just over five years ago, Dr. Xu and I and our colleagues developed a bold vision,” says Holmes. “We believed we could work towards curing then-untreatable diseases by creating new technologies and by partnering with some of the best minds in neuroscience, genomics, virology, biomedical engineering, computer science, and mathematics.”
That vision, he notes, was built on the belief that collaboration—not just within labs, but across disciplines—could unlock therapeutic avenues once thought unimaginable. The BEC-targeting AAV tool is not an endpoint. It’s a door, newly opened.
Rewriting the Playbook: Targeting Excitatory Neurons
While one arm of the Armamentarium project focuses on the vasculature, another has plunged into the complex world of excitatory neurons—cells responsible for transmitting signals throughout the brain. These neurons play vital roles in memory, decision-making, spatial navigation, and overall cognitive function.
In a second paper, published in Cell Reports Methods under the title “An AAV capsid proposed as microglia-targeting directs genetic expression in forebrain excitatory neurons,” a new surprise emerged. Researchers found that an AAV vector previously believed to target microglia—another type of brain cell involved in immune response—actually showed strong specificity for excitatory neurons in the forebrain.
This serendipitous discovery, led by graduate researcher Wenhao Cao, challenges assumptions about how AAVs interact with brain cell types.
“Our group found that AAV-MG1.2 actually achieves specific genetic expression in an entirely different brain cell type: excitatory neurons in the forebrain region across different animal species,” says Cao.
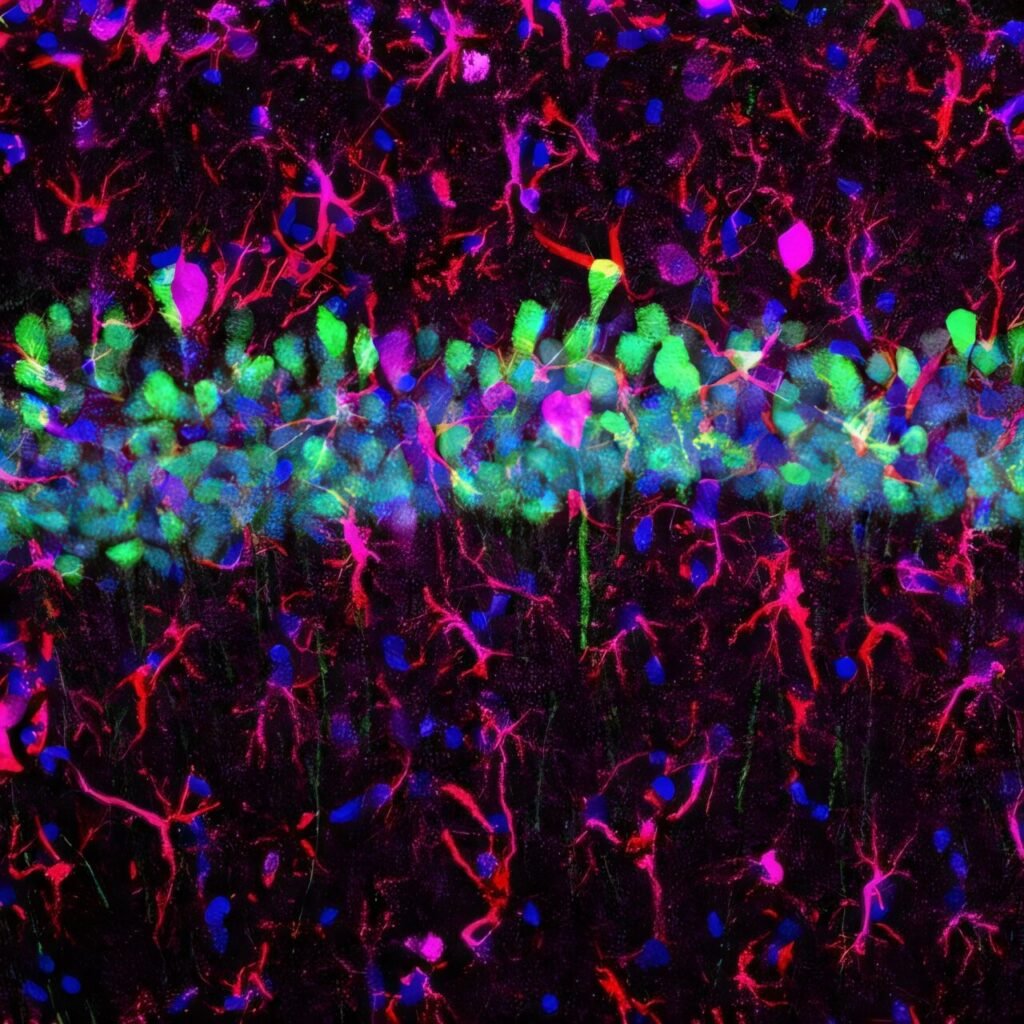
Disproving Assumptions, Building Tools
Rather than being a disappointment, this unexpected result became a revelation. Excitatory neurons are among the most functionally significant cells in the brain—and among the hardest to reach with gene therapy tools. The researchers quickly pivoted to characterize and validate AAV-MG1.2’s potential.
They found that AAV-MG1.2 could reliably target forebrain excitatory neurons in a variety of animal models, from mice to primates. Moreover, when combined with specially designed enhancer elements, the vector could selectively express therapeutic genes in subpopulations of excitatory neurons—providing exquisite control over where and how a therapy acts.
“Our study expands the existing toolbox for targeting excitatory neurons,” says Cao. “AAV-MG1.2 opens up a promising avenue for exploring specific brain cell subtypes, which could eventually lead to clinical applications that facilitate targeted therapy.”
This tool could be used not only for therapeutic gene delivery but also for mapping brain circuits. Neural circuit tracing—identifying which neurons are connected to which others—is essential for understanding brain disorders ranging from epilepsy to autism.
What We Still Don’t Know
Despite the success, mysteries remain. Why does AAV-MG1.2 favor excitatory neurons? What molecular cues does it follow to gain entry and activate genes specifically in those cells?
“The biological mechanism underlying AAV-MG1.2’s specific targeting of excitatory neurons remains unclear,” says Cao. “Elucidating this mechanism will be critical for expanding the toolbox of AAV-based tools used in neuroscience research.”
If researchers can understand these mechanisms, they may be able to rationally design future AAVs for even more precise targeting—down to individual cell types or even disease states. Imagine an AAV vector that turns on only in malfunctioning cells, or that seeks out regions of early pathology before symptoms emerge. That future is now within reach.
From Vectors to Vision: A New Era of Neurotherapeutics
Taken together, the studies from the Armamentarium consortium do more than just introduce two new AAV tools. They represent a broader shift in how neuroscience approaches the brain—not as an inaccessible fortress, but as a complex landscape that can be navigated, understood, and ultimately healed.
The implications reach far beyond academic interest. Neurological diseases are among the most devastating and intractable conditions in medicine. Alzheimer’s disease alone affects over 50 million people worldwide and currently has no cure. Stroke is a leading cause of disability. Disorders of cognition and memory rob people of their identities.
By enabling precise, noninvasive delivery of therapeutic genes to the brain’s vascular system and cognitive control centers, the new AAV tools could be the first step toward transformative treatments. And perhaps, one day, cures.
A Science of Collaboration, A Future of Possibility
None of this happened in isolation. These breakthroughs were the product of teams willing to cross disciplines, challenge assumptions, and embrace the unpredictable nature of discovery.
Dr. Xu’s lab at UC Irvine served as the intellectual nexus, but contributions came from institutions across California and beyond. The Allen Institute for Brain Science brought deep expertise in neural circuits. UC San Diego researchers contributed genomic and computational insights. And the results have reverberated across the scientific world.
It is no exaggeration to say that these advances may redefine how we treat brain disease. What once required invasive surgery, risky procedures, or systemic drugs may soon be possible through a simple intravenous injection—powered by AAV vectors that know exactly where to go.
The brain, long considered medicine’s final frontier, is opening itself to a new kind of exploration. And the tiny viral vehicles leading the way are doing more than just delivering DNA. They are delivering hope.
References: Eric Velazquez-Rivera et al, Specific targeting of brain endothelial cells using enhancer AAV vectors, Neuron (2025). DOI: 10.1016/j.neuron.2025.03.031
Wenhao Cao et al, An AAV capsid proposed as microglia-targeting directs genetic expression in forebrain excitatory neurons, Cell Reports Methods (2025). DOI: 10.1016/j.crmeth.2025.101054