In the fight against obesity and type 2 diabetes, few drugs have made waves like semaglutide. This medication, already revolutionizing weight management, belongs to a class of compounds known as GLP-1 receptor agonists (GLP-1R agonists). These drugs mimic the hormone GLP-1 (glucagon-like peptide-1), enhancing insulin release, suppressing appetite, and promoting fat loss. While semaglutide has delivered remarkable outcomes for many patients—often leading to significant and sustained weight loss—it comes with a catch: unwanted side effects, including nausea and muscle wasting.
Now, in a groundbreaking new study published in Cell Metabolism, scientists at the Sahlgrenska Academy at the University of Gothenburg have dissected the drug’s mechanism with remarkable precision. Using cutting-edge neuroscience techniques in mice, they’ve not only pinpointed the specific brain cells responsible for semaglutide’s positive effects, but they’ve also shown it may be possible to separate these benefits from the side effects. This discovery may reshape how we understand—and refine—some of today’s most powerful metabolic therapies.
A Revolution in a Needle: Semaglutide’s Rise
Originally developed to treat type 2 diabetes, semaglutide has become a cornerstone in obesity treatment. It operates by mimicking GLP-1, a gut hormone released after eating. GLP-1 plays a vital role in enhancing insulin secretion, slowing gastric emptying, and reducing hunger by acting on the brain. These effects are mediated by GLP-1 receptors, which are found in both the pancreas and the central nervous system.
But while the benefits have turned semaglutide into a household name among weight-loss drugs—bolstered by media attention and celebrity endorsements—the experience isn’t always smooth. Patients often report gastrointestinal discomfort, ranging from mild nausea to vomiting. More concerningly, some users also experience muscle mass reduction, raising questions about long-term health impacts.
Until now, scientists haven’t fully understood why semaglutide works so well for appetite suppression and fat loss, nor why it causes these side effects. The new research from Sahlgrenska Academy brings fresh clarity.
Into the Mouse Mind: Mapping Drug Effects in the Brain
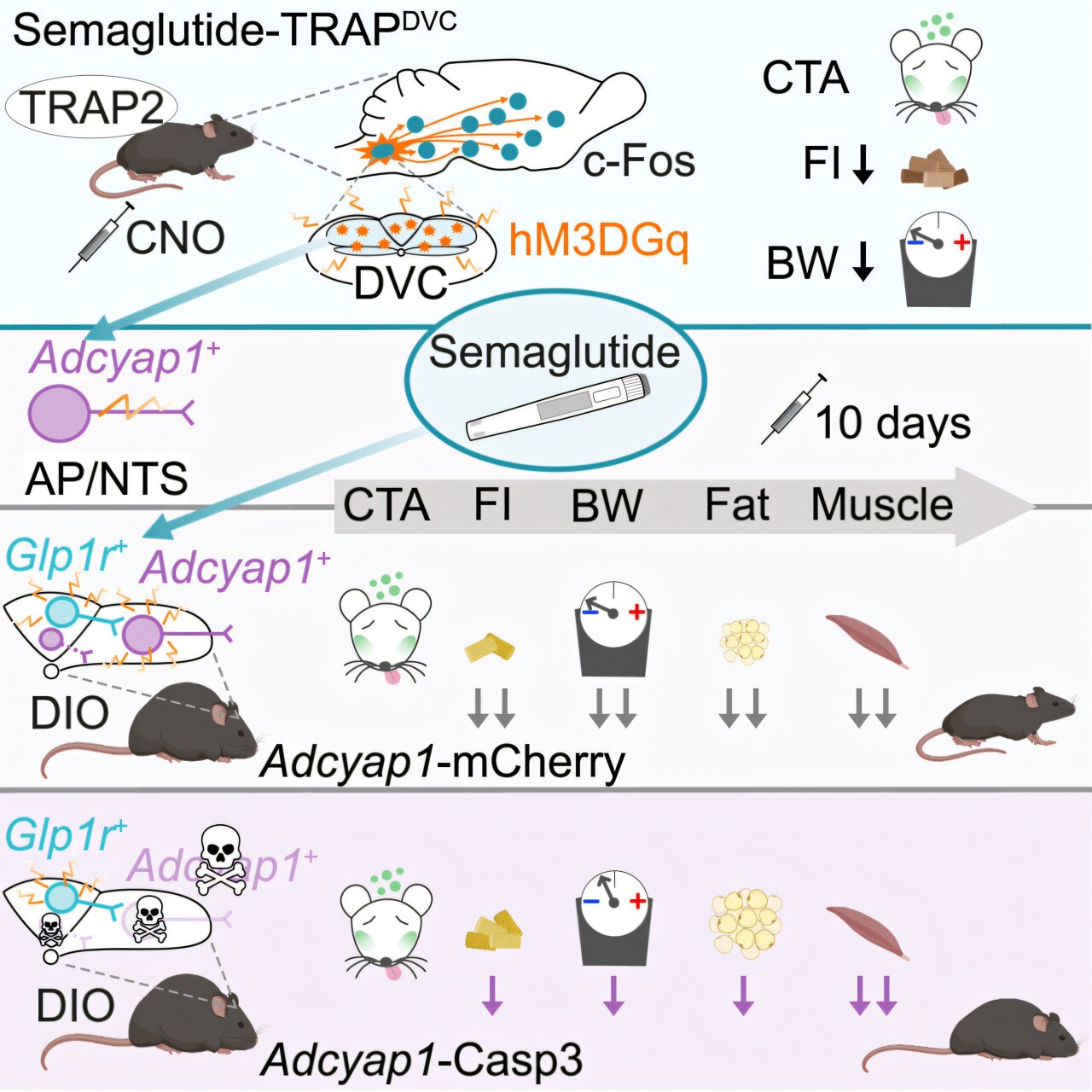
To unravel semaglutide’s neural targets, researchers led by Ph.D. student Júlia Teixidor-Deulofeu, and under the guidance of Dr. Linda Engström Ruud, turned to laboratory mice. Rather than administering semaglutide indiscriminately, the team used a strategy that allowed them to track and manipulate specific nerve cells activated by the drug.
These nerve cells turned out to be located in a small but vital region of the brainstem known as the dorsal vagal complex (DVC). This area, situated near the medulla oblongata, acts as a key hub for regulating hunger, satiety, and energy balance. Importantly, the DVC is also directly influenced by signals from the gut, making it a natural target for GLP-1-related activity.
Using optogenetics and chemogenetics—techniques that use light or engineered receptors to activate or silence neurons—the scientists could turn on the same neurons that semaglutide normally activates, but without using the drug itself. The outcome was striking: mice still ate less and lost weight, even without semaglutide in their systems.
More tellingly, when these specific neurons were destroyed or silenced, semaglutide’s appetite-suppressing and fat-reducing effects diminished drastically. But here’s the twist: nausea-like symptoms and muscle loss still occurred, even in the absence of those particular nerve cells.
Decoding Benefit from Burden
This finding is more than just a quirk of neurobiology. It demonstrates, for the first time, that the neural pathways responsible for semaglutide’s weight loss effects are distinct from those involved in side effects. In other words, the drug’s helpful and harmful impacts are separable at the level of the brain.
“This suggests that these nerve cells control the beneficial effects of semaglutide,” said Teixidor-Deulofeu. “We have therefore identified a specific group of nerve cells that is necessary for the effects that semaglutide has on weight and appetite, but which does not appear to contribute to any significant extent to side effects such as nausea.”
In theory, a future version of semaglutide—or a next-generation therapy—could be designed to target only these beneficial neural circuits, bypassing the areas responsible for negative side effects.
Beyond Obesity: Broader Applications and Deeper Questions
The implications extend far beyond weight loss. GLP-1R agonists like semaglutide are already being investigated for an astonishing range of conditions, from addiction and alcohol use disorder to Alzheimer’s and Parkinson’s diseases. This is because GLP-1 receptors are found not only in appetite circuits, but in other regions of the brain involved in reward, memory, and cognition.
Yet, the lack of clarity about how GLP-1R agonists function in the brain has limited these possibilities. This study provides a roadmap for precision targeting—offering scientists a way to isolate the specific effects they want without the baggage.
“It is important to understand how these drugs actually work. The better we understand this, the greater the opportunity we have to improve them,” said Dr. Engström Ruud.
What Comes Next?
While the study was conducted in mice, the neural architecture between rodents and humans in brainstem circuits is strikingly conserved, particularly in areas like the dorsal vagal complex. This raises the possibility that similar strategies could eventually be used in humans.
The next steps will likely involve identifying the molecular signatures of these specific neurons—what proteins they express, how they communicate, and whether their activity can be controlled pharmacologically or through gene therapy. There’s also interest in whether people who are more sensitive to semaglutide’s side effects have differences in how these neural circuits are organized.
Pharmaceutical companies have already begun developing “second-generation” GLP-1 drugs that combine the hormone with other peptides, or use dual agonists to amplify benefits. But this new research suggests that understanding the exact brain wiring may be the key to making these treatments safer, smarter, and more effective.
The New Frontier in Appetite Science
Obesity and metabolic disorders are among the most pressing health challenges of our time. For many, semaglutide has offered hope—and dramatic results—where diet, exercise, and willpower alone have failed. But the future of these treatments lies not just in stronger drugs, but in more intelligent ones.
By dissecting the brain’s response at the cellular level, scientists are now poised to engineer therapies that fine-tune the body’s energy balance with unprecedented precision. This is no longer just about weight loss. It’s about harnessing the brain’s own circuitry to fight disease—a strategy that could reshape the next generation of metabolic and neurological medicine.
Thanks to this study, the once-blurred neural map behind semaglutide’s effects is coming into sharp focus. And in the folds of the brainstem, researchers have found not only answers, but a path forward.
Reference: Júlia Teixidor-Deulofeu et al, Semaglutide effects on energy balance are mediated by Adcyap1+ neurons in the dorsal vagal complex, Cell Metabolism (2025). DOI: 10.1016/j.cmet.2025.04.018