Beneath the surface of our skin, in the blood that rushes through our veins, and in the trillions of cells that make us human, lies a breathtaking secret. Every single cell in the human body houses an instruction manual almost two meters long—our DNA. Yet, miraculously, this long, fragile string of genetic code is compacted into a space smaller than a speck of dust. It is here, in this invisible world, that the architecture of life unfolds, and sometimes, where it unravels.
This miracle of biological organization is possible because DNA isn’t just floating freely in the cell nucleus—it is elegantly, meticulously coiled around microscopic protein spools. These structures, known as nucleosomes, are the master organizers of the genome. Like thread wrapped tightly around bobbins, DNA is looped and folded again and again until it fits inside a volume of just a few hundred cubic micrometers.
But this tightly packed beauty comes with a cost. Hidden deep within this orderly labyrinth lie genetic instructions that must be read and executed constantly—orders that control growth, development, and repair. If those orders are locked away, if the cell cannot access them when danger strikes, the consequences can be catastrophic. This is especially true when it comes to one of the most important defenders in the cellular army: a protein called p53.
The Guardian of the Genome
Known as “the guardian of the genome,” p53 is a sentinel standing watch over our genetic material. Its role is to patrol the cell, detect DNA damage, and respond swiftly. Sometimes, it repairs damage. Other times, when the damage is too extensive, p53 pulls the kill switch, ordering the cell to self-destruct in a process called apoptosis. In this way, p53 protects us from the transformation of normal cells into cancerous ones.
But p53’s power is fragile. In more than half of all human cancers, p53 is mutated, suppressed, or hijacked. When this guardian falls silent, cells begin to grow uncontrollably, ignoring the rules that normally keep them in check. This makes understanding p53 one of the central challenges in cancer biology. Scientists have spent decades studying how it works, when it activates, and—most puzzlingly—how it finds its targets.
The challenge is this: most of the DNA sequences p53 must access are buried deep within nucleosomes. These tightly packed structures wrap DNA in ways that make much of it inaccessible. So how does p53 do its job? How does it find and interact with these hidden sites in a genome that resembles a dense jungle more than a neat filing cabinet?
A Puzzle Inside a Maze
This question has long haunted molecular biologists. Imagine a city of a trillion doors, many of them locked or hidden behind walls, and a single detective trying to find the one room where a crime is taking place. That’s p53 inside the nucleus. It’s not enough to simply know the sequence of DNA. You must understand the architecture—the physical folding, bending, and wrapping of that sequence into chromatin, the full package of DNA and protein.
This complex spatial arrangement has made it difficult for scientists to determine how p53 physically navigates this terrain. Adding to the mystery, p53 doesn’t act alone. It has molecular allies and enemies—other proteins that either help stabilize it or mark it for destruction. Understanding how all of these molecules interact in the tangled web of chromatin has remained one of the most pressing and elusive goals in cancer research.
Until now.
Uncovering the Molecular Choreography
A team led by Dr. Nicolas Thomä, a cancer biologist at the École Polytechnique Fédérale de Lausanne (EPFL), has opened a new window into this secret world. Their study, published in the journal Molecular Cell, reveals a previously hidden layer of control in how p53 operates within the chromatin jungle. By combining cryo-electron microscopy, genome-wide mapping, and molecular assays, the researchers reconstructed in astonishing detail how p53 interacts with DNA even when it’s locked within nucleosomes.
Cryo-electron microscopy—an advanced imaging technique that freezes molecules in action—allowed the scientists to visualize the actual binding events between p53 and DNA at near-atomic resolution. What they discovered was both surprising and illuminating.
Rather than being entirely blocked by nucleosomes, p53 finds windows of opportunity. It binds especially well at the outer edges of nucleosomes, where DNA begins to wind or unwind from the protein spool. These “entry” and “exit” points offer p53 enough exposure to grasp its DNA targets. It’s like slipping a hand through the edge of a curtain rather than tearing it down.
But that was only the beginning.
Friends, Enemies, and the Architecture of Access
The study went further, asking whether other proteins—p53’s molecular companions and adversaries—could also access it when it was bound to nucleosomal DNA. Two proteins were of special interest. The first, USP7, is a cellular ally of p53. It stabilizes the guardian, extending its lifespan and giving it time to repair damage. The second, a viral complex known as E6-E6AP, is an enemy. Originating from the human papillomavirus (HPV), this complex seeks to neutralize p53, paving the way for uncontrolled cell growth and, eventually, cancer.
To the researchers’ astonishment, they found that USP7 could successfully reach and bind to p53 even when p53 was latched onto nucleosomal DNA. The bond was stable, enduring, and visible using cryo-EM. In contrast, the E6-E6AP complex could not access p53 under the same conditions. The architecture of the chromatin—the very physical form of the genome—was acting as a gatekeeper.
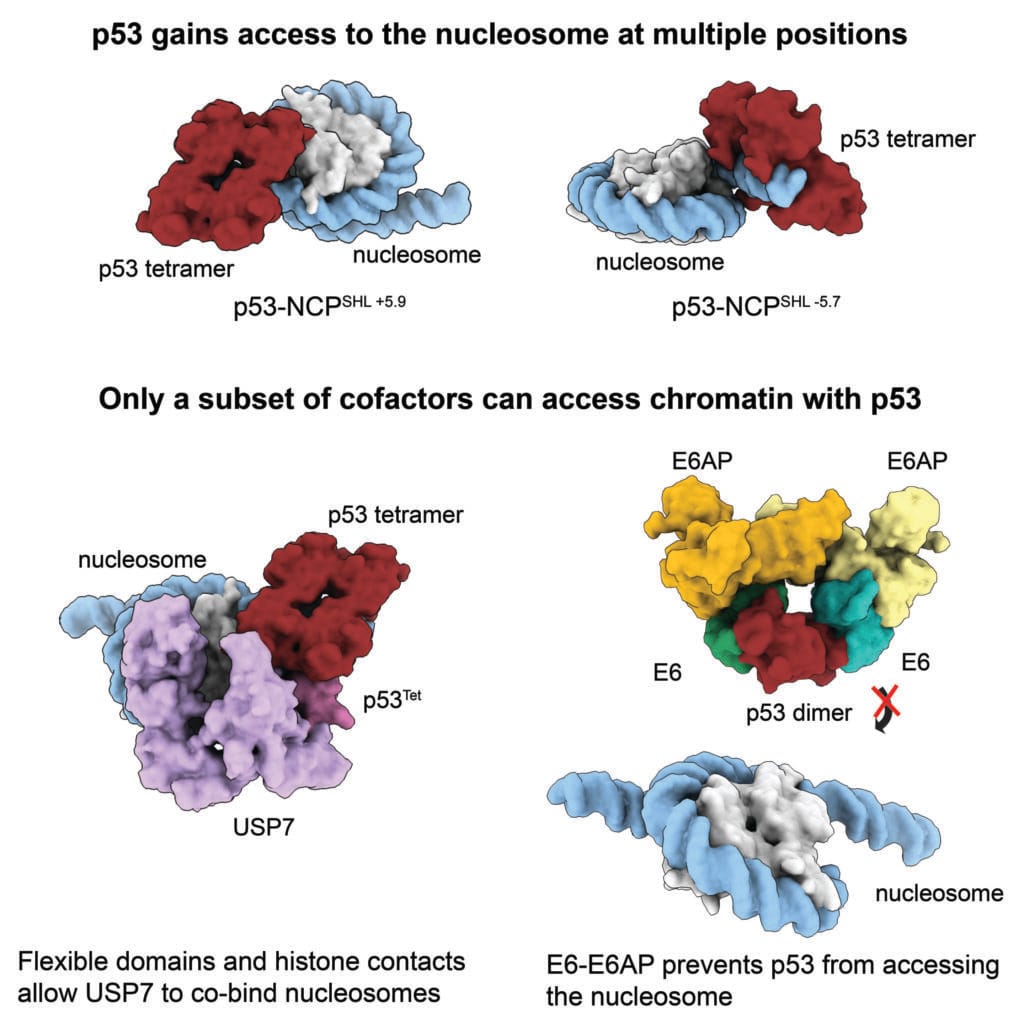
This finding was profound. It meant that nucleosomes don’t just store genetic information; they actively control how and when molecular conversations can happen. The way DNA is wrapped determines who can speak to whom. It’s as if the genome itself has built-in security doors, allowing friendly interactions while blocking intruders.
A Hidden Layer of Cellular Intelligence
What Thomä and his team uncovered is not just a detail about p53. It is a larger truth about how life functions at the molecular level. The genome isn’t just a code—it’s a three-dimensional, dynamic landscape, one that shapes behavior not just through chemical signals, but through physical form. Nucleosomes, once thought to be passive packaging units, are now revealed as intelligent regulators of access and control.
This discovery adds a crucial piece to the puzzle of cancer biology. If certain harmful proteins cannot reach p53 while it’s bound to chromatin, but beneficial ones can, then therapies could potentially exploit this difference. Perhaps we could design drugs that help maintain p53’s interactions with helpful cofactors while shielding it from destructive ones.
Moreover, the research opens the door to understanding how other key cellular proteins behave under similar conditions. p53 is not alone in working within the context of nucleosomes. Most transcription factors—proteins that turn genes on or off—must operate within this same maze. By deciphering how structure influences function, scientists may finally begin to decode the deeper language of cellular decision-making.
Toward a Future of Smarter Medicine
What does all this mean for patients? For those facing the terrifying diagnosis of cancer, or for the millions who carry p53 mutations in their cells, these findings offer something long overdue: a better map.
Current cancer therapies often take a brute-force approach—bombarding cells with chemicals or radiation to induce damage. But understanding the nuanced choreography of molecules within chromatin could lead to a new kind of medicine—one that is smarter, more precise, and deeply rooted in the real architecture of life.
Imagine being able to enhance p53’s function by modifying chromatin structure to favor beneficial interactions. Or blocking harmful ones without disabling the guardian itself. These aren’t science fiction dreams—they’re real possibilities born from understanding the hidden gatekeeping function of nucleosomes.
A Dance in the Dark, Finally Illuminated
The cell is a place of lightless miracles, a place where molecules dance without our witness. Yet in those quiet, hidden movements, life is made, sustained, and defended. What Thomä’s team has done is bring light into that darkness—not just metaphorically, but literally, freezing and capturing these molecular events for us to see.
Their work is a reminder that in biology, structure is not separate from function—it is function. And even in the tiniest spaces—smaller than a millionth of a milliliter—vast and beautiful systems of control are at work, keeping us alive.
p53, the ever-watchful guardian, now has its story expanded. We understand better how it moves, how it speaks, and how it is protected. And with this understanding comes a promise: that the mysteries of cancer are not impenetrable, that the doors we once thought locked may in fact be openable—if only we know where to look.
Reference: Deyasini Chakraborty et al, Nucleosomes specify co-factor access to p53, Molecular Cell (2025). DOI: 10.1016/j.molcel.2025.06.027