Inside every living cell, there is a constant hum of activity so familiar to biologists that it can be easy to overlook its mysteries. DNA is copied, messages are passed along, proteins are built, and life continues its intricate choreography. Yet sometimes, the most transformative discoveries emerge not from distant frontiers, but from unexpected signals hidden within well-known genetic territory. In a recent study from researchers at Texas A&M University Health Science Center, such a signal came into focus, revealing an RNA molecule that quietly holds together one of the cell’s most vital structures.
Published in the Proceedings of the National Academy of Sciences, the research describes the discovery of a previously unknown non-coding RNA that preserves the integrity of the nucleolus, a central hub of cellular activity. Beyond its fundamental biological role, this RNA also appears to influence survival outcomes in certain blood cancers, offering a striking example of how basic molecular biology can intersect with human disease.
When RNA Refuses to Follow the Script
For decades, the role of RNA seemed well defined. It was understood as a short-lived messenger, copied from DNA and sent off to guide the production of proteins. In this framework, genes existed primarily to encode proteins, and RNA served as a temporary courier, delivering instructions and then fading away.
The new study challenges this tidy narrative. The researchers identified an RNA molecule that does not become a protein at all. Instead, it acts directly within the cell nucleus, regulating essential structures without ever entering the protein-making pipeline. This class of molecules, known as non-coding RNAs, has been gaining attention, but this discovery adds a particularly unexpected twist.
The RNA, named CUL1-IPA, originates from the well-characterized CUL1 gene, long known for producing a protein involved in cellular processes. What surprised the researchers was that the same gene could also give rise to an RNA with an entirely different mission. Rather than leaving the nucleus to help build proteins, CUL1-IPA stays behind, quietly supporting the architecture and function of the nucleolus.
“This finding redefines the conventional assumption that protein-coding genes produce only protein-related messages,” said Irtisha Singh, Ph.D., senior author of the study.
The Nucleolus, Held Together by RNA
The nucleolus is a dense region within the nucleus, and its importance cannot be overstated. It is the site where ribosomes are produced, the molecular machines responsible for assembling proteins. Without a functioning nucleolus, cells struggle to survive, let alone grow.
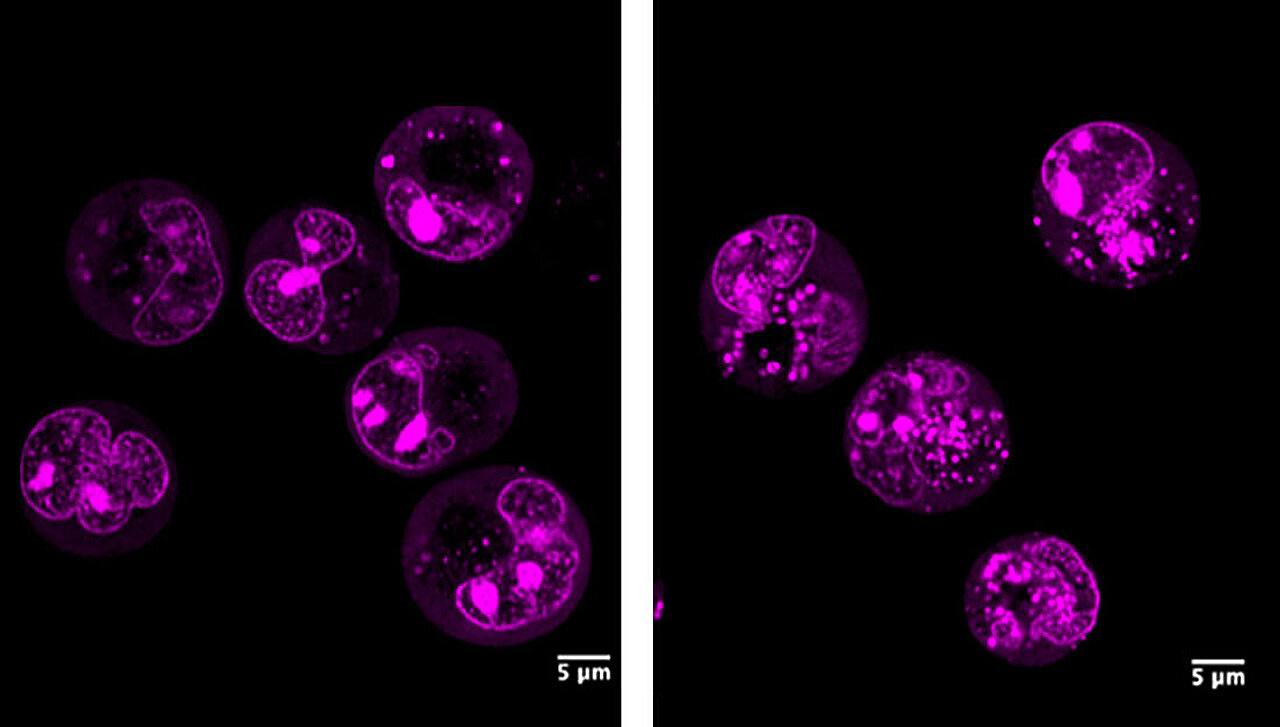
To understand what CUL1-IPA was doing, the researchers removed it from living cells. The results were immediate and dramatic. The nucleolus began to break apart, losing its structural integrity. Cells showed clear signs of stress, indicating that something fundamental had been disrupted.
“We were amazed at how essential this RNA turned out to be,” said Sumana Mallick, Ph.D., co-first author of the study. “Removing it caused the nucleolus to lose its structural integrity, making it clear that non-coding RNAs from protein-coding genes can play central regulatory roles.”
The finding revealed that CUL1-IPA is not a minor molecular byproduct. It is a key structural and functional supporter of the nucleolus, ensuring that ribosome production proceeds smoothly. In other words, this RNA helps maintain the cell’s ability to manufacture proteins by safeguarding the very factory that builds the machinery.
A Molecular Clue in Blood Cancers
The implications of this discovery extend well beyond the laboratory bench. The research team examined patient data from two types of blood cancers, multiple myeloma and chronic lymphocytic leukemia, searching for patterns linked to CUL1-IPA.
What they found was striking. Patients with more severe forms of these cancers had higher levels of CUL1-IPA, regardless of how much of the traditional CUL1 RNA was present. The association was not subtle. Elevated levels of this non-coding RNA correlated with patient survival, suggesting that it may play a role in how aggressively these cancers behave.
“Its expression correlates with patient survival in blood cancers and may contribute to how aggressive these cancers become,” said Pranita Borkar, Ph.D., co-first author of the article.
The connection makes biological sense within the context of the study’s findings. Cancer cells are known for their rapid growth, and rapid growth demands intense ribosome production. By supporting the nucleolus, regulatory RNAs like CUL1-IPA may inadvertently help cancer cells sustain the high levels of protein synthesis they need to proliferate.
One Gene, Many Voices
Perhaps the most profound message of this research lies in how it reshapes our understanding of genes themselves. The discovery of CUL1-IPA adds to a growing body of evidence that genes are far more versatile than once believed. A single gene is not limited to producing a single message or performing a single role.
Instead, one stretch of DNA can generate multiple RNA molecules, each with distinct functions. Some become proteins, while others remain as RNA, influencing cellular structures and regulatory networks in ways that are only now coming into view. This layered complexity challenges long-standing assumptions and invites scientists to look more closely at what else might be hidden within familiar genes.
The work from the Singh Lab highlights how much remains to be learned, even about genetic regions that have been studied for years. It also underscores the importance of non-coding RNAs as active participants in cellular life, not mere background noise in the genome.
Why This Discovery Matters
At its core, this research reveals a new way in which cells maintain their internal organization and survive under stress. By identifying CUL1-IPA as a guardian of nucleolar integrity, the study deepens our understanding of how fundamental cellular structures are regulated.
The clinical implications are equally compelling. Because CUL1-IPA levels correlate with survival in certain blood cancers, this RNA may serve as a biomarker, helping clinicians better understand disease severity. In the future, molecules like CUL1-IPA could even become therapeutic targets, opening the door to treatments that disrupt cancer cells by undermining their ability to maintain a functional nucleolus.
More broadly, the discovery encourages a shift in how scientists think about genes and RNA. It suggests that critical regulators of health and disease may be hiding in plain sight, embedded within genes long assumed to have a single purpose. By listening more carefully to these quiet molecular voices, researchers may uncover new strategies to diagnose, understand, and ultimately treat complex diseases.
In revealing the unexpected power of a single RNA molecule, this study reminds us that even in the most familiar corners of biology, there are still stories waiting to be told.
Study Details
Sumana Mallick et al, Intronic polyadenylation–derived long noncoding RNA modulates nucleolar integrity and function, Proceedings of the National Academy of Sciences (2026). DOI: 10.1073/pnas.2514521123