At the very start of life, before there is an organism, before there is even a recognizable cell with a destiny, something extraordinary happens. A group of cells gathers around a future egg, not to compete, but to give everything away. These cells, known as nurse cells, will eventually empty their entire contents into a single developing egg cell. Every organelle, every protein, every piece of internal machinery is transferred. Nothing is held back.
“This is a very dramatic process that is foundational to the development of life,” said Wen Lu, Ph.D., a research assistant professor of Cell and Developmental Biology and co-author of a new study from Northwestern Medicine. For decades, scientists have known this transfer must happen for a healthy egg to form. What remained a mystery was how cells manage such an extreme and perfectly coordinated internal reorganization without collapsing into chaos.
Now, a study published in the Journal of Cell Biology has illuminated the hidden architecture behind this cellular handoff. By watching egg development unfold inside Drosophila melanogaster, researchers uncovered an intricate partnership between two of the cell’s most important structural systems, revealing how life’s earliest construction project is carefully built from the inside out.
The Cell’s Skeleton Comes Alive
Every cell has an internal framework known as the cytoskeleton, a dynamic network that gives the cell shape, strength, and direction. Two major components of this framework are actin filaments and microtubules. Actin filaments are flexible and adaptable, allowing cells to bend, contract, and reshape themselves. Microtubules are stiffer, forming long, straight tracks that guide transport and help organize the cell’s interior.
Scientists have long understood that these two systems interact. What they did not understand was how they coordinate during complex developmental moments, especially during the creation of an egg cell, when timing and precision are everything.
“This is a classic example of what we call ‘crosstalk’ in biology,” said Vladimir Gelfand, Ph.D., the Leslie B. Arey Professor of Cell, Molecular, and Anatomical Sciences and co-senior author of the study. Crosstalk describes the way different cellular systems communicate, influence one another, and work together toward a shared outcome.
The Northwestern team set out to watch that conversation unfold in real time.
Watching the Scaffold Rise
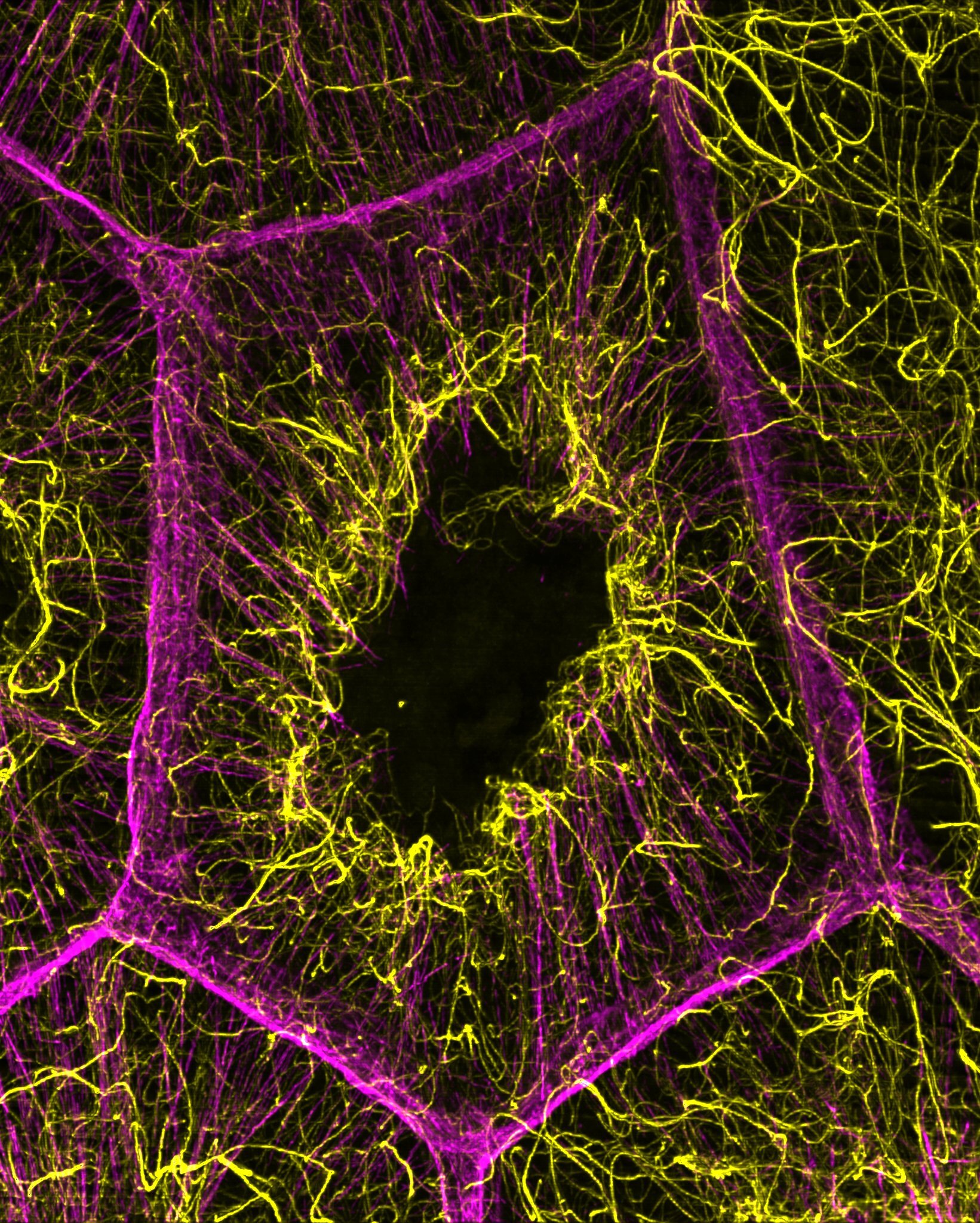
Using advanced microscopy, the researchers focused on the nurse cells surrounding the developing egg. What they saw was a carefully timed construction sequence. As actin cables began to emerge, the nurse cells were simultaneously building a network of microtubules that was not only extensive but chemically reinforced.
These microtubules were acetylated, meaning they had undergone a chemical modification that made them more stable. This stability turned out to be essential. When researchers disrupted the acetylated microtubule network, the consequences were immediate and dramatic. The actin cables failed to form properly. They could neither initiate nor elongate as they normally would.
This finding revealed something unexpected. Rather than actin filaments leading the process, the microtubules were laying down a foundational scaffold first. The microtubules were not passive tracks waiting to be used. They were active organizers, defining where and how actin structures could grow.
In the context of egg development, this scaffold is crucial. Actin cables play a key role in moving cellular contents toward the egg. Without properly formed actin cables, the nurse cells cannot complete their act of self-emptying. The egg, deprived of essential materials, cannot develop normally.
When Support Becomes a Dialogue
The story did not end with microtubules guiding actin. The researchers discovered that the relationship ran both ways. When key actin-bundling proteins were lost, the microtubule network itself began to suffer. There were fewer microtubules, and those that remained were shorter and less organized.
This reversal was a crucial clue. It showed that actin filaments are not simply following instructions from microtubules. Instead, bundled actin actively helps maintain microtubule spacing and orientation. Actin provides flexibility, while microtubules provide rigidity. Together, they strike a balance that allows the cell to be both stable and adaptable during the intense demands of egg development.
The researchers describe this as a true partnership. Each system influences the assembly, positioning, and organization of the other. Neither can function properly alone. Together, they create the internal architecture needed to move vast amounts of material into the forming egg without breaking the cell apart.
A Cellular Transfer Like No Other
The sheer scale of what happens during this stage of development makes the findings even more striking. Nurse cells do not send a few molecules or select components. They send everything. Their cytoplasm, packed with vital materials, flows into the egg cell in a coordinated surge.
For this to work, the internal scaffolding must be flawless. Microtubules need to be strong enough to maintain structure, yet arranged in a way that allows movement. Actin cables need to grow in the right places, at the right time, and with the right length. Any misstep could derail the entire process.
By revealing how acetylated microtubules and actin filaments cooperate, the study explains how cells meet this challenge. It shows that egg development is not driven by a single dominant structure but by an ongoing conversation between systems, each adjusting to the other as conditions change.
Seeing the Invisible More Clearly
One of the most exciting aspects of the work lies not only in what was discovered, but in how it was discovered. The team relied on cutting-edge microscopy tools that allowed them to visualize cellular structures with remarkable clarity. These tools made it possible to see stable microtubule networks forming in real time alongside growing actin cables.
“We are very lucky to have access to some of the best equipment in the world to help answer these fundamental scientific questions,” Lu said.
Looking ahead, the researchers hope to push these techniques even further. By visualizing microtubules in greater detail, they aim to uncover even finer aspects of how cellular architecture is built and maintained during development.
Why This Quiet Cellular Conversation Matters
At first glance, a study of fruit fly egg development might seem distant from everyday life. Yet the implications reach far beyond a single species. As Gelfand noted, many aspects of egg development are conserved across species, meaning the basic principles uncovered here apply broadly in biology.
Understanding how actin filaments and microtubules coordinate gives scientists deeper insight into one of life’s most fundamental processes: the creation of a healthy egg. This knowledge helps explain how cells manage extreme internal reorganization without losing function or integrity.
More broadly, the study offers a powerful reminder of how life is built. Not through isolated parts working alone, but through constant communication, adjustment, and cooperation at the smallest scales. Inside a developing egg, invisible structures negotiate, support, and correct one another, ensuring that life begins with a solid foundation.
In revealing this hidden dialogue, the Northwestern Medicine team has brought us closer to understanding how life organizes itself from the very first moments, when everything depends on a perfectly timed cellular conversation.
Study Details
Wei-Chien Chou et al, Developmentally regulated actin–microtubule cross talk in Drosophila oogenesis, Journal of Cell Biology (2026). DOI: 10.1083/jcb.202411007