For most of their existence, fungi live unnoticed lives. They break down fallen leaves, recycle nutrients, and quietly keep ecosystems running. Many are harmless companions of plants, animals, and soil. Yet a new study suggests that for some fungi, the line between a peaceful existence and a dangerous one may be thinner than anyone expected.
Researchers from Kiel University and the Max Planck Institute for Evolutionary Biology set out to understand how certain fungi manage to adapt to the human body. Their findings reveal a surprising truth: the transformation from harmless environmental organism to emerging human pathogen may not require new genes or exotic weapons. It may only require learning how to use what is already there, faster and more efficiently.
As global temperatures rise and fungal infections increase worldwide, this discovery reshapes how scientists think about fungal threats to human health.
A World Warming, and Fungi Taking Notice
Fungi are everywhere, and most of them are not dangerous. They decompose organic matter, release nutrients into soil, and support entire ecosystems. Some even live in close partnership with multicellular organisms, offering benefits to their hosts. But under certain conditions, a subset of fungi becomes opportunistic pathogens, especially when the immune system is weakened.
The rise in global temperatures has coincided with an increase in fungal infections affecting crops, wildlife, and humans. This trend has pushed researchers to look more closely at fungi that were once considered benign. Could some of these organisms be quietly evolving the ability to thrive inside the human body?
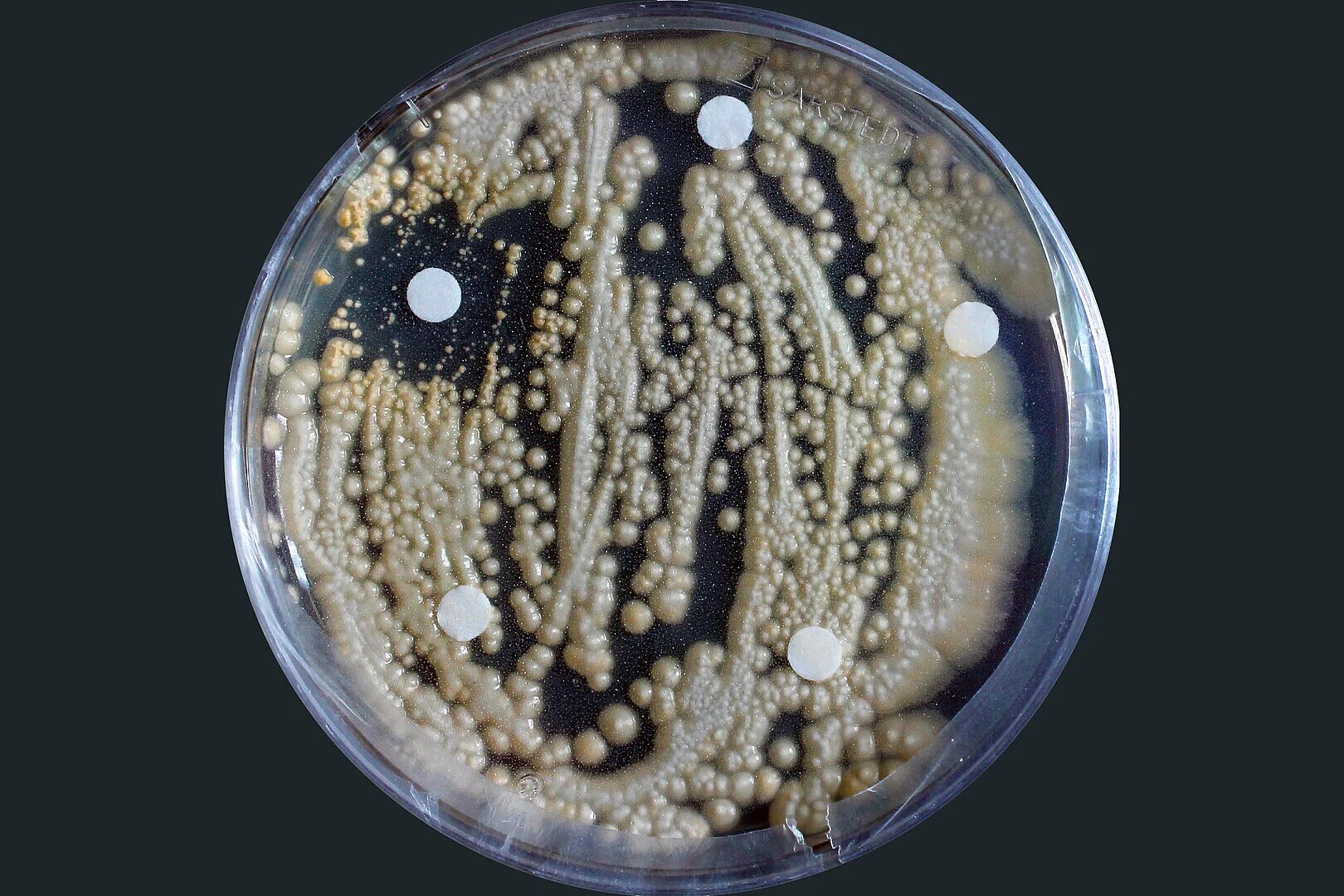
To explore this question, a team led by Professor Eva Stukenbrock, head of the Environmental Genomics group at Kiel University and MPI-EvolBio, focused on fungi belonging to the order Trichosporonales. This group includes both soil-dwelling species and species capable of causing serious infections in humans. The contrast offered a rare opportunity to see what truly separates the harmless from the harmful.
Looking for Villains, Finding Something Else
The researchers began with a straightforward assumption. Dangerous fungi, they believed, must carry special genes that allow them to attack human cells, produce toxins, or evade the immune system. These so-called virulence genes have long been considered the hallmark of pathogenic microbes.
But when the team compared the genomes of harmless and pathogenic Trichosporonales, the expected differences did not appear.
“We were surprised,” explains Dr. Marco Guerreiro, the study’s first author. The genetic makeup of both groups was remarkably similar. The harmful fungi did not possess a dramatically different set of genes. They were, in genetic terms, close cousins of their soil-dwelling relatives.
This raised an unsettling question. If the genes were largely the same, what was allowing some species to flourish inside the human body while others remained harmless?
The Hidden Power of Protein Production
The answer turned out to lie not in the genes themselves, but in how efficiently those genes are used. The key difference was protein production efficiency, a late step in gene expression known as translation.
In all living cells, genes are first copied into messenger RNA, or mRNA. These mRNA molecules are then translated into proteins, the working molecules that carry out nearly all cellular functions. Translation depends on small molecular matchmakers called tRNA, which read short sequences in the mRNA known as codons and assemble the correct amino acids into proteins.
In the pathogenic fungi, this system was finely tuned. Their codons and tRNA molecules matched exceptionally well, allowing proteins to be produced faster and more efficiently. This was not a general speed boost affecting all proteins equally. Instead, the optimization was focused on proteins involved in fat metabolism.
This detail turned out to be crucial.
Learning to Live on Fat
The human body is rich in lipids, or fats. Skin, organs, and tissues provide a fat-heavy environment that is dramatically different from soil, where fats are scarce and carbon-rich compounds dominate. For a fungus accustomed to life in the ground, the human body is a foreign landscape.
The pathogenic fungi studied had adapted to this challenge by optimizing their ability to metabolize fat. By producing fat-processing proteins more quickly, they could adjust rapidly to the lipid-rich conditions inside mammals.
“Soil-living fungi tend to specialize in a carbon-centered lifestyle,” says Guerreiro. “Pathogenic fungi, in contrast, have developed a strategy that lets them thrive where lipids are abundant.”
This ability to rapidly retool their metabolism may be what allows these fungi to cross the threshold into pathogenicity. They are not fundamentally different organisms. They are simply better prepared to take advantage of the human body’s resources.
Evolution at the Speed of Translation
The study suggests that adaptive evolution has acted on both codon usage and tRNA composition in pathogenic fungi. Over time, this fine-tuning improves how efficiently key proteins are produced, without altering the underlying gene content.
This insight changes how scientists think about the evolution of fungal pathogens. Instead of requiring dramatic genetic innovations, pathogenic potential can emerge through subtle shifts in efficiency. A fungus does not need new genes to become dangerous. It only needs to use existing genes more effectively in the right environment.
To test whether this idea held up beyond genomic data, the researchers moved into the laboratory.
Proof in the Lab
In controlled experiments, fungi with genes optimized for fat metabolism were grown in lipid-rich conditions. The results mirrored the genomic predictions. These fungi adapted faster and grew significantly better than their soil-oriented relatives.
The laboratory findings confirmed that enhanced protein production linked to fat metabolism is not just a theoretical advantage. It provides a real, measurable benefit in environments similar to the human body.
This experimental validation strengthened the team’s conclusion that efficient translation plays a central role in enabling fungi to adapt to mammalian hosts.
A Smaller Step Than We Thought
One of the most striking implications of the study is how small the evolutionary step toward pathogenicity appears to be. Because harmless and harmful species share such similar genetic foundations, the barrier separating them may be much lower than previously assumed.
This raises concerns about fungi that currently thrive at human body temperature but are not yet known to cause disease. With only modest changes in protein production efficiency, such species might gain the ability to colonize human tissues.
The situation becomes even more troubling when viewed alongside the growing problem of antifungal drug resistance. If new pathogens emerge more easily, and existing treatments become less effective, managing fungal diseases could become increasingly difficult.
Watching the Future Take Shape
Recognizing these risks, the researchers aim to identify fungal species that carry genomic signatures associated with pathogenic potential. By spotting these warning signs early, scientists hope to monitor and possibly mitigate future threats before they become widespread health problems.
The study, published in Nature Communications with collaborators from Braunschweig, Bochum, and Illinois, highlights the need for a broader perspective on fungal evolution. Pathogenicity is not a fixed trait but a dynamic possibility shaped by environment, efficiency, and opportunity.
Why This Research Matters Now
This research fundamentally changes how fungal pathogenicity is understood. It shows that the transition from harmless environmental organism to human health threat may be faster and more accessible than anyone imagined.
As climate change alters ecosystems, as populations of immunocompromised individuals grow, and as global connectivity increases, fungi are being presented with new opportunities to explore new hosts. Understanding the subtle evolutionary mechanisms that enable this shift is no longer an academic exercise. It is a matter of public health.
By revealing that efficiency, rather than genetic novelty, can drive the emergence of fungal pathogens, this study offers both a warning and a guide. It warns that danger may be closer than expected, hidden in organisms we have long ignored. And it guides researchers toward new ways of identifying and monitoring potential threats before they emerge.
In a warming world, even the quietest soil dwellers may be listening closely to the signals of the human body, ready to adapt.
Study Details
Marco Alexandre Guerreiro et al, Genomic and physiological signatures of adaptation in pathogenic fungi, Nature Communications (2026). DOI: 10.1038/s41467-026-68330-6