Imagine walking into a bakery and being instantly enveloped by the comforting aroma of fresh bread. Or catching the faint scent of a rose that transports you to a summer garden. Smell is one of the most powerful and emotional senses we possess—it can evoke memories, alter mood, and even influence behavior. Yet for all its importance, the biology of smell has remained one of the least understood frontiers in human science.
Humans have around 400 odorant receptors (ORs)—specialized proteins in the nose that detect odor molecules and send signals to the brain. These receptors are the gateway to our olfactory world, translating invisible molecules in the air into rich sensory experiences. But despite decades of research, scientists have only been able to match a small fraction of these receptors with the specific molecules they detect.
Until recently, only 71 human receptor-ligand interactions—pairings between odor molecules and their receptors—had been confidently identified. Even more puzzling, many of these known interactions showed weak responses in lab tests, leaving researchers uncertain about how our brains truly recognize and distinguish smells. For years, it was like trying to understand a language while knowing only a handful of its words.
The Old Theory of a Complex Code
In 2004, the field of olfactory research seemed to gain clarity. The Nobel Prize that year honored scientists Richard Axel and Linda Buck for their groundbreaking work on the sense of smell. They proposed what became known as the “combinatorial model” of olfaction—a theory that revolutionized how scientists thought about scent detection.
According to the combinatorial model, each odor is not detected by just one receptor but by a unique combination of many. Just as letters combine to form words, multiple receptors together create a specific pattern that the brain interprets as a particular smell. This model explained how humans could detect thousands of different scents with only a few hundred receptors—an elegant solution to a complex problem.
However, while the model remains powerful, it also left lingering questions. Was every scent really the product of multiple receptors working together? Or could some smells be recognized by a single receptor—like a solo instrument playing a clear, distinct note amid the orchestra of the nose?
A Breakthrough from Switzerland
A recent study published in Current Biology offers striking new insights that may reshape this understanding. A team of Swiss researchers developed a novel method that finally overcame one of olfactory science’s biggest technical hurdles: getting human odorant receptors to function properly in lab conditions.
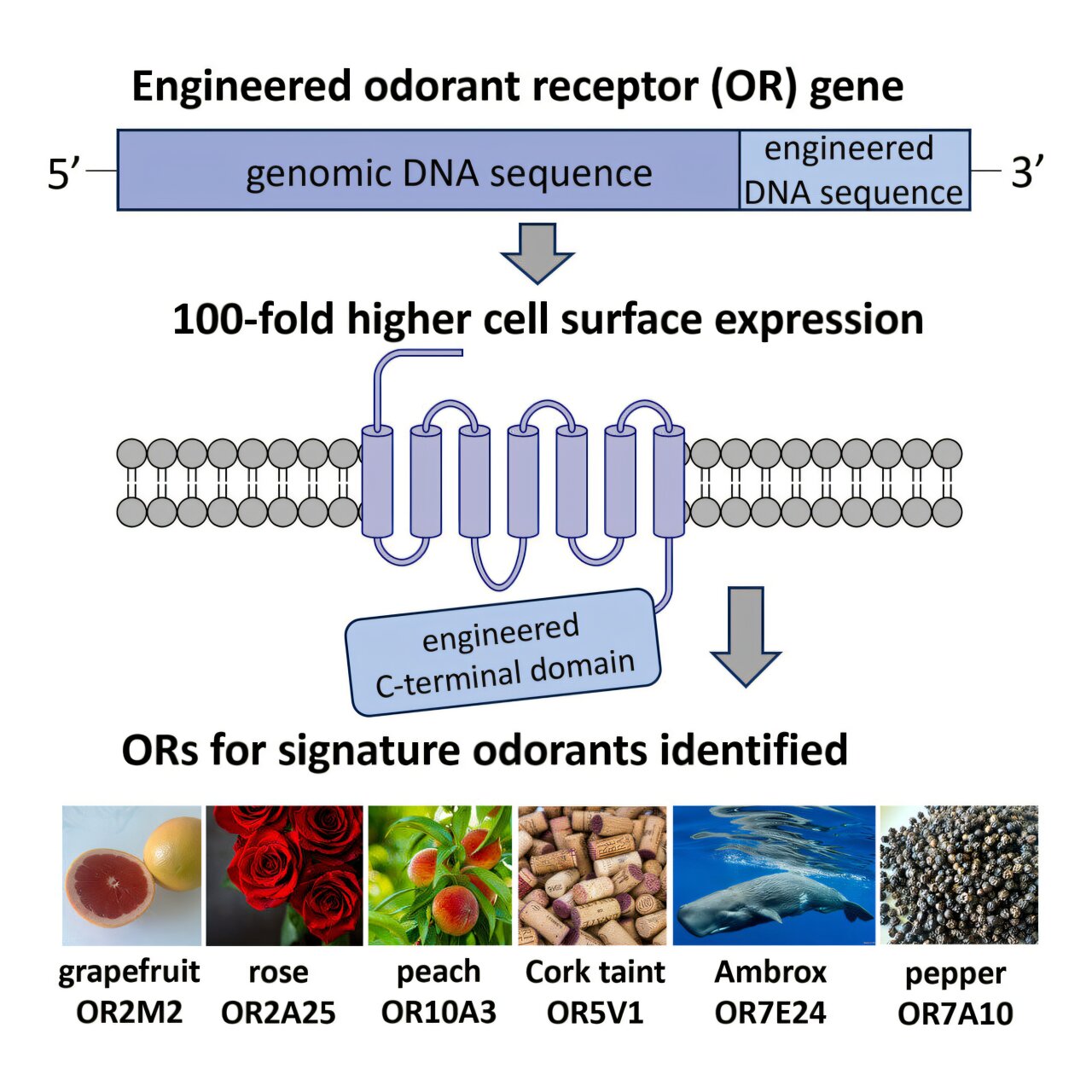
Typically, when scientists try to study these receptors outside the body, the proteins fail to express correctly on the surface of cells. Without proper expression, the receptors can’t interact with odor molecules effectively, making it nearly impossible to measure which scents they respond to.
The Swiss researchers tackled this problem by tweaking a specific part of the receptor structure—the C-terminal domain. By modifying this region, they dramatically boosted the receptors’ ability to reach the cell surface and respond to odorants. Suddenly, the once-silent receptors came alive, responding robustly to scents in ways that had never been observed before.
This new approach allowed the team to test a wide range of smells, from the earthy sweetness of ambergris and the floral notes of rose to the creamy warmth of vanilla and the sharp tang of corked wine. What they discovered would challenge long-held assumptions about how we experience scent.
De-Orphanizing the Receptors
For decades, many human odorant receptors have been considered “orphans”—proteins with no known ligand, or matching odor molecule. The Swiss team’s breakthrough method allowed them to “de-orphanize” several of these receptors, meaning they were finally able to identify which molecules bind to which receptors.
And the results were remarkable. Not only did they find new receptor-ligand pairings, but the sensitivity of their tests far exceeded anything seen before.
Previous studies reported a median EC50 (a measure of how strongly a receptor responds to an odor) of about 10⁻⁴ M. In contrast, the new data showed a median of 1.6 × 10⁻⁶ M—roughly a hundredfold improvement in sensitivity. This means the receptors could detect odor molecules at concentrations far lower than previously thought possible, a closer reflection of how the human nose actually works in real life.
When One Receptor Rules Them All
Perhaps the most fascinating finding from this research was that some odors appear to rely on just one dominant receptor. While the combinatorial model remains valid for many scents, this new data revealed that certain signature smells—those that we recognize instantly and describe with familiar words like “woody,” “musky,” or “vanilla”—may be primarily detected by a single receptor with high specificity.
For instance, the researchers found that a receptor known as OR7A17 plays a major role in detecting woody odors. Previous studies had suggested that a molecule called Arborone, known for its woody scent, might bind to as many as ten different receptors. But the new evidence showed that one receptor—OR7A17—was both highly sensitive and sufficient for detecting this scent on its own.
Similarly, receptors such as OR2M2, OR2A25, and OR10G3 were found to correspond strongly to specific odor categories like “grapefruit,” “rosy,” and “vanilla.” What’s more, each of these receptors could respond to chemically diverse molecules that shared the same odor profile. This finding suggests that the brain’s perception of certain smells may depend more on how the receptor interprets an odor’s structural essence than on its precise chemical formula.
These results hint that human olfaction, at least for distinct signature scents, might operate more like a pharmacological system—where a single ligand binds tightly to a specific target receptor—than a purely combinatorial one.
Rethinking How the Brain Sees Smell
This new view doesn’t completely overturn the combinatorial model but rather refines it. It suggests that while complex odors—like coffee, perfume, or wine—may still activate a mosaic of receptors, simpler or more defining scents can be recognized by a single receptor acting as a “master key.”
In other words, our sense of smell may operate along a spectrum: sometimes relying on a chorus of receptor signals, other times guided by the clarity of one dominant note.
This could also explain why certain smells feel so immediate and recognizable. That flash of memory when you catch a whiff of sunscreen or cinnamon? It might be because your brain doesn’t need to process a complex pattern—it knows exactly which receptor has fired and what that means.
The Human Element: Why This Matters
At first glance, this might seem like a purely scientific curiosity—a step forward in the chemistry of scent. But the implications reach far beyond the laboratory. Understanding how specific odorant receptors detect certain smells could revolutionize industries from perfumery to medicine, food science, and even environmental monitoring.
If we can pinpoint exactly how receptors identify specific odor molecules, we could design artificial noses—devices that mimic the human olfactory system—to detect food spoilage, monitor air quality, or identify hazardous gases long before humans could.
In the culinary world, chefs and flavor scientists could craft foods with precise aromatic profiles, engineered to trigger particular sensory responses. In healthcare, better knowledge of OR–ligand interactions could help diagnose or treat conditions like anosmia—the loss of smell—or even influence how medications are perceived by the senses.
The Challenges That Remain
Despite these advances, the researchers acknowledge that their work is just the beginning. Even with enhanced receptor expression and sensitivity, lab assays can’t fully replicate the complex environment of the human nose. In our bodies, odorant receptors exist within specialized tissues, surrounded by mucus, enzymes, and other cellular components that influence how scents are detected and processed.
Further optimization of these techniques could help scientists express more of the remaining orphan receptors and improve the accuracy of in vitro models. Bridging the gap between laboratory data and the true human experience of smell remains one of biology’s greatest challenges.
The Poetry of Perception
There’s something poetic about this science. Smell is the most ancient of our senses—an invisible thread connecting us to the world and to our memories. Long before sight or sound evolved to their current complexity, primitive organisms used chemical cues to find food and avoid danger. In that sense, our noses carry an evolutionary legacy billions of years old.
Every time we inhale, we engage in a delicate conversation with the molecules around us. They enter our nostrils, bind to receptors, and spark a cascade of signals that become sensations, emotions, and memories. The smell of the sea, the scent of rain on dry earth, the perfume of someone you love—these are not just experiences; they are stories told in molecules.
The work of the Swiss researchers brings us closer to understanding how these stories are written and read. It bridges chemistry and consciousness, offering a glimpse into how something as invisible as a molecule can evoke something as powerful as nostalgia or joy.
Toward a New Understanding of Smell
The human sense of smell has long been underestimated, seen as primitive compared to vision or hearing. Yet this new research reminds us that olfaction is both exquisitely precise and deeply emotional—a sense that binds biology and feeling in ways we are only beginning to understand.
As scientists continue to decode the genetic and molecular language of scent, we stand on the edge of a new era of sensory science. One where we may not only comprehend how we perceive the world’s smells but also learn to recreate, enhance, or even design them from scratch.
In unlocking the secrets of our odorant receptors, we are doing more than solving a biochemical puzzle—we are uncovering the intimate architecture of one of humanity’s most evocative senses. Each discovery brings us closer to understanding how invisible molecules shape our experiences, our memories, and, in a way, our very humanity.
Smell, it turns out, is not just a sense. It is a bridge between the physical and the emotional, between matter and meaning—a whisper from the molecular world reminding us what it feels like to be alive.
More information: Roger Emter et al, Decoding human olfaction by high heterologous expression of odorant receptors detecting signature odorants, Current Biology (2025). DOI: 10.1016/j.cub.2025.09.041