For doctors and scientists, one of the most haunting questions in medicine never fully goes away. Why does cancer sometimes return even after treatment seems to work? Why do some bacteria stubbornly survive powerful antibiotics that should have wiped them out? The answers often feel slippery, as if survival were governed by chance rather than rules.
At the heart of this mystery lies something scientists call biological noise. Inside every living cell, countless chemical reactions are happening at once. Even when cells share the same genes, the amounts of proteins they produce can differ wildly. These random fluctuations create rare “outlier” cells that quietly escape drugs and treatments. They survive, multiply, and later emerge as resistant cancer cells or antibiotic-defying bacteria.
For years, researchers could control only the average behavior of large groups of cells. The unpredictable individuality of single cells remained beyond reach. Now, a team of researchers believes they have found a way to tame that randomness itself, not by suppressing life’s complexity, but by mathematically guiding it.
The Trap of Averages and the Cells That Slip Through
Cells are built to survive. They try to maintain internal balance, or homeostasis, even while their environments constantly change. Modern genetic circuit technologies can adjust protein production so that, on average, a population of cells behaves as desired. But averages can be dangerously misleading.
The research team described this problem with a vivid analogy. Controlling only the average protein level is like setting a shower to 40°C and assuming everything is fine. In reality, the water might be rapidly switching between boiling hot and freezing cold. Even though the average temperature is perfect, the experience is unbearable.
Cells behave the same way. When genetic circuits regulate only the average, individual cells can swing to extremes. A small fraction of cells escapes control entirely. These escapees become the seeds of relapse and resistance. This phenomenon, which the researchers called the “trap of the average,” has long limited progress in cancer treatment and synthetic biology.
What scientists needed was a way to control not just the mean behavior of cells, but the variability itself. Until now, that challenge had remained unsolved.
A Mathematical Lens on Cellular Chaos
The breakthrough came from a collaboration between Professor Jae Kyoung Kim of KAIST, Professor Jinsu Kim of POSTECH, and Professor Byung-Kwan Cho of KAIST. Instead of starting with biological experiments, they began with mathematics.
Their goal was to establish what they called a noise control principle. Using mathematical modeling, they sought a way to eliminate biological noise and precisely guide the fate of individual cells. The idea was bold: treat randomness not as an unavoidable feature of life, but as something that could be designed and controlled.
This approach led them to explore how cells might sense their own fluctuations. They focused first on a process called a dimerization reaction, where final products bind together to form pairs. Through theoretical analysis, they found that dimerization could act as a sensor, detecting noise in the cellular state. It could tell when protein levels were fluctuating too much.
But sensing the noise was not enough. Early attempts showed that while dimerization could detect variability, it could not eliminate the differences between cells on its own. The problem of runaway fluctuations remained.
Building a Controller That Acts in Real Time
To truly suppress noise, the researchers realized that cells needed a way to respond instantly when protein levels became excessive. This insight led them to combine noise sensing with a second principle: degradation-based actuation.
In simple terms, this mechanism breaks down proteins as soon as they are overproduced. By pairing this rapid degradation with noise detection, the team designed a new mathematical model they called the noise controller, or NC.
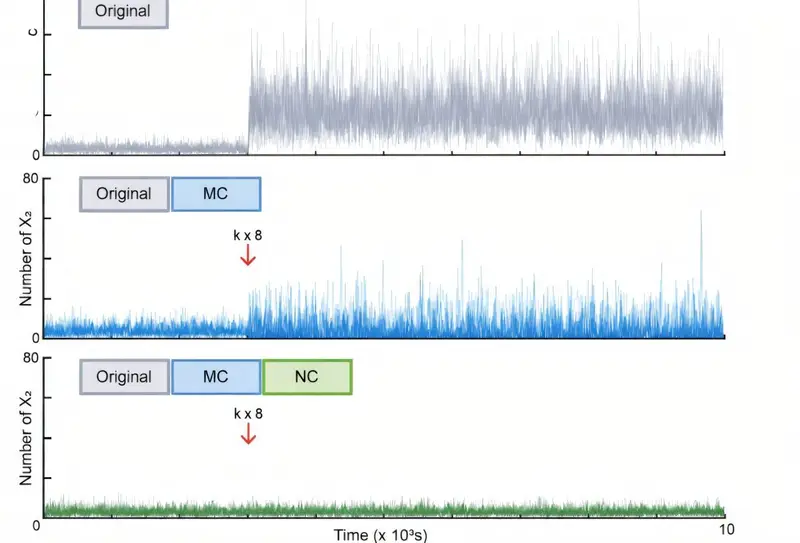
This controller did something remarkable. It achieved what the researchers described as noise robust perfect adaptation, or Noise RPA. Even when external conditions changed, the level of noise remained constant. The fluctuations that once allowed rogue cells to slip through were held firmly in check.
Through this design, the team theoretically suppressed cell-to-cell variability to a Fano factor of 1. This represents the minimum level of noise achievable by universal biological systems. In effect, they had found the mathematical limit of how orderly living systems can be, and showed how to reach it.
Testing the Idea in a Virtual Cell
To demonstrate the power of their model, the researchers applied it virtually to a DNA repair system in E. coli. In the existing biological system, the results were grim. Protein levels varied so much between individual cells that about 20% of them failed to repair their DNA and died.
When the noise controller was applied, the picture changed dramatically. By unifying protein levels across all cells, the mortality rate dropped to 7%. Without altering the average behavior of the system, the team had significantly improved survival simply by reducing variability.
What makes this result striking is how it was achieved. No new drugs were introduced. No biological components were physically altered. The improvement came entirely from sophisticated mathematical principles that reshaped how the system behaved.
This shift marks a departure from population-level control toward true single-cell precision. Each cell is treated not as part of a statistical blur, but as an individual whose fate can be guided.
Turning Luck Into Design
For decades, biological noise was often dismissed as bad luck or coincidence. Some cells survived treatment, others did not, and that randomness was accepted as inevitable. This research challenges that assumption at its core.
Professor Jae Kyoung Kim captured this transformation clearly when he said, “The significance lies in bringing cellular noise—which was previously dismissed as luck or coincidence in biological phenomena—into the realm of controllable factors through mathematical design. It will play a vital role in fields requiring precise cellular control, such as overcoming cancer treatment resistance and developing high-efficiency smart microorganisms.”
The work also highlights the power of mathematics as a creative force in biology. Professor Jinsu Kim of POSTECH emphasized this point, stating, “This research demonstrates the power of mathematical modeling, starting from theoretical formulas of intracellular noise using reaction network theory and leading to the design of actual biological mechanisms.”
The study, published in Nature Communications, stands as a theoretical milestone. It shows that randomness inside cells is not an unbreakable barrier, but a challenge that can be met with careful design.
Why This Research Matters
At its core, this work reframes how we think about control in living systems. Diseases like cancer and the rise of antibiotic resistance are often driven by rare cells that escape treatment. These cells are not necessarily stronger or smarter; they are simply different, shaped by fluctuations that push them outside the reach of average-based therapies.
By offering a way to suppress those fluctuations, this research points toward a future where treatments could be designed to leave no hiding place for outliers. Instead of hoping that drugs catch most cells, scientists could aim for precision that reaches every single one.
Beyond medicine, the implications extend to synthetic biology, where controlling cellular behavior with high accuracy is essential. Smart microorganisms designed for industrial or environmental purposes could become more reliable and efficient when noise is no longer left unchecked.
Most importantly, this research sends a powerful message. Even in systems as complex and seemingly unpredictable as living cells, order can emerge from careful thought. What once looked like chance can become design. And in that transformation lies the possibility of turning some of humanity’s most stubborn biological challenges into solvable problems.
More information: Dongju Lim et al, Toward single-cell control: noise-robust perfect adaptation in biomolecular systems, Nature Communications (2025). DOI: 10.1038/s41467-025-67736-y