In 2020, a scientific revelation sparked fascination across the world: the Moon, our silent companion in the night sky, appeared to be rusting. Researchers had detected hematite, an iron oxide mineral better known as rust, spread across the higher latitudes of the lunar surface—particularly on the side that always faces Earth.
This finding baffled scientists. Rust forms when iron reacts with oxygen and water, but the Moon is notoriously dry and nearly devoid of oxygen. With no protective atmosphere of its own, and constantly bombarded by solar wind carrying hydrogen—the very element that usually prevents rusting—the presence of hematite seemed almost impossible. How, then, could Earth’s barren and airless neighbor be developing something so dependent on oxygen?
Searching for an Explanation
When the news first broke, several theories were put forward. Perhaps oxygen came from lunar volcanic gases, released long ago during the Moon’s active geological past. Maybe asteroids or comets delivered it in small amounts during impacts. Some even suggested large collisions could have temporarily created oxygen-rich environments.
Yet none of these ideas could fully explain the specific distribution of the rust. Why was hematite concentrated more heavily on the Moon’s nearside, the hemisphere that faces Earth? Why did it appear predominantly at higher latitudes? These patterns hinted that something else was influencing the Moon’s chemistry—something directly tied to Earth.
Earth Wind: A Hidden Connection Between Worlds
The key breakthrough came when scientists turned their attention to Earth’s magnetosphere, the giant magnetic bubble that surrounds our planet and shields us from the Sun’s harmful radiation. Normally, the solar wind—a steady stream of charged particles from the Sun—dominates the space around the Moon, showering it with hydrogen ions. But for about five days each month, when the Earth lies directly between the Sun and the Moon, something remarkable happens.
During this alignment, the Moon slips into Earth’s magnetotail, the elongated shadow of the magnetosphere. Here, streams of oxygen ions from Earth’s upper atmosphere are carried outward into space, and some of them rain down onto the lunar surface. Scientists call this phenomenon “Earth wind.”
This mechanism, they realized, could provide the missing oxygen. When Earth wind delivers its charged oxygen ions to the Moon’s iron-rich soil, oxidation can occur—even without water. The puzzle of lunar rust suddenly began to make sense.
Testing the Theory in the Laboratory
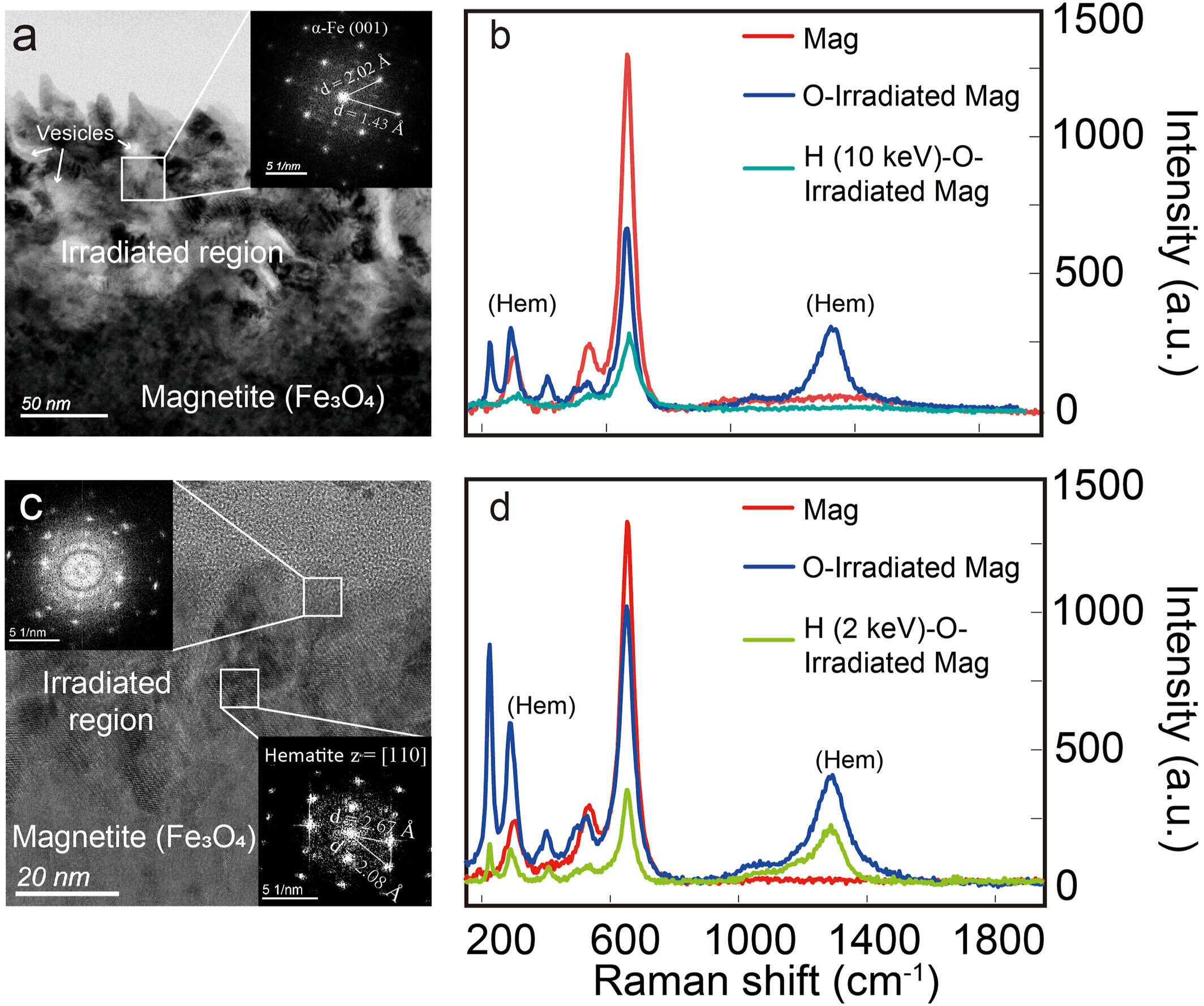
Although the Earth wind explanation was elegant, scientists needed more than theory—they needed experimental proof. A recent study published in Geophysical Research Letters set out to recreate lunar conditions in the laboratory and put the idea to the test.
The research team gathered iron-bearing minerals commonly found in lunar soil, including metallic iron, iron sulfide, and ilmenite. They then exposed these samples to streams of oxygen ions and hydrogen ions at energies similar to those found in space. Some experiments mimicked Earth wind, while others simulated the relentless solar wind.
The results were striking. Oxygen ions, just like those thought to arrive from Earth, successfully oxidized the lunar minerals, creating detectable layers of hematite. The Moon, it seemed, really could rust when exposed to oxygen carried on the cosmic winds of our own planet.
The Tug-of-War Between Rust and Reduction
Yet another question lingered: if solar wind hydrogen constantly batters the Moon, wouldn’t it undo the process? Hydrogen acts as a reducing agent, stripping oxygen away and restoring iron to its metallic form. If that were the case, hematite shouldn’t persist on the surface.
The researchers tested this by irradiating hematite with both high-energy and low-energy hydrogen ions. They found that high-energy hydrogen—similar to what sometimes arrives in Earth wind—was capable of reversing the rust, turning hematite back into iron. Low-energy hydrogen from solar wind, however, was far less effective. Its shallow penetration into the mineral meant that it couldn’t fully reach and erase the oxidized layers.
This delicate balance suggests that the survival of hematite on the Moon depends not just on the presence of oxygen but also on the type and energy of hydrogen ions interacting with the soil. It is a constant tug-of-war between oxidation and reduction, with Earth wind occasionally tipping the balance toward rust formation.
Why the Rust Appears at the Moon’s Poles
The study also helped explain why hematite is more abundant in the Moon’s higher latitudes. These regions are more exposed to Earth wind particles when the Moon passes through the magnetotail. In addition, traces of water molecules have been detected in the lunar polar regions, likely trapped in shadowed craters and soil. Even tiny amounts of water can accelerate oxidation, reinforcing the role of the poles as hotspots for lunar rust.
Implications for Planetary Science
The discovery of hematite on the Moon does more than reveal an unexpected chemical process. It highlights the profound and ongoing connection between Earth and its closest neighbor. Earth is not just shaping the Moon through gravitational tides or orbital dynamics—it may also be directly influencing its chemistry by delivering oxygen across space.
This finding forces planetary scientists to rethink how celestial bodies interact. If Earth’s atmosphere can “spill over” onto the Moon, could similar processes occur elsewhere in the solar system? Could magnetospheres and atmospheric escape shape the surfaces of moons around Jupiter, Saturn, or even exoplanets in distant star systems?
The study also raises questions about the future. As humans prepare for renewed exploration of the Moon, including potential long-term bases, understanding how its surface chemistry evolves will be critical. Rust may not pose a structural threat, but it carries clues about the Moon’s environment, Earth’s influence, and the broader dynamics of space weather.
The Continuing Mystery
While the new experiments provide strong support for the Earth wind theory, they are not the final word. Laboratory conditions can only approximate the complexity of the lunar environment. Factors like micrometeorite impacts, variations in solar activity, and subtle geological processes may all play roles in the Moon’s evolving surface chemistry.
Future lunar missions, equipped with advanced instruments, may directly measure the interactions between Earth wind and lunar soil. These studies could confirm not only how hematite forms but also how it survives in the face of competing forces.
A Poetic Link Across Space
In the end, the story of lunar rust is about more than minerals and particles. It is a reminder of the intimate bond between Earth and the Moon. Our atmosphere stretches farther than we might imagine, reaching across 384,000 kilometers to touch the lunar soil. In a sense, the Moon carries a fingerprint of Earth, etched into its surface in red streaks of rust.
The sightless companion that has guided our calendars, tides, and dreams for millennia is not as separate from us as it appears. Its chemistry tells a story of invisible winds that connect worlds, of oxygen carried across the void, of Earth shaping its neighbor in quiet but profound ways.
The Moon rusts because of us—because of Earth’s breath in space. And in that rust lies a testament to the strange, beautiful, and sometimes unexpected ways the universe binds us together.
More information: Xiandi Zeng et al, Earth Wind‐Driven Formation of Hematite on the Lunar Surface, Geophysical Research Letters (2025). DOI: 10.1029/2025gl116170