Inside every human cell lies a fortress—a dense, well-guarded chamber known as the nucleus. Within its walls, genetic secrets are stored, cell fates are decided, and life is regulated at its deepest level. For most invaders, the journey ends at the nuclear border, where the gates, called nuclear pores, are notoriously selective. But HIV-1 is not most invaders.
For decades, scientists have asked how this virus—one of the deadliest pathogens in human history—manages to infiltrate the nucleus so stealthily, so precisely. What gives it the key to unlock this heavily guarded gate?
Now, thanks to the groundbreaking work of Professor Peijun Zhang and her team at the University of Oxford and the UK’s National Electron Bio-Imaging Centre (eBIC), a vital part of the answer has finally come into view—quite literally. Through the lens of cryo-electron microscopy, researchers have caught HIV-1 in the act of breaching the nuclear barrier. The details of this long-hidden step in the viral life cycle are not only visually stunning but scientifically game-changing.
A Clearer View of a Killer
The technique used to uncover these secrets—cryo-electron microscopy (cryo-EM)—has revolutionized modern biology. By flash-freezing cells and imaging them at near-atomic resolution, cryo-EM allows scientists to view biological molecules in action without destroying their natural context. In this study, researchers used cell-permeabilization to gently open the outer membranes of cells, keeping their inner architecture intact while allowing the virus to behave almost exactly as it would during real infection.
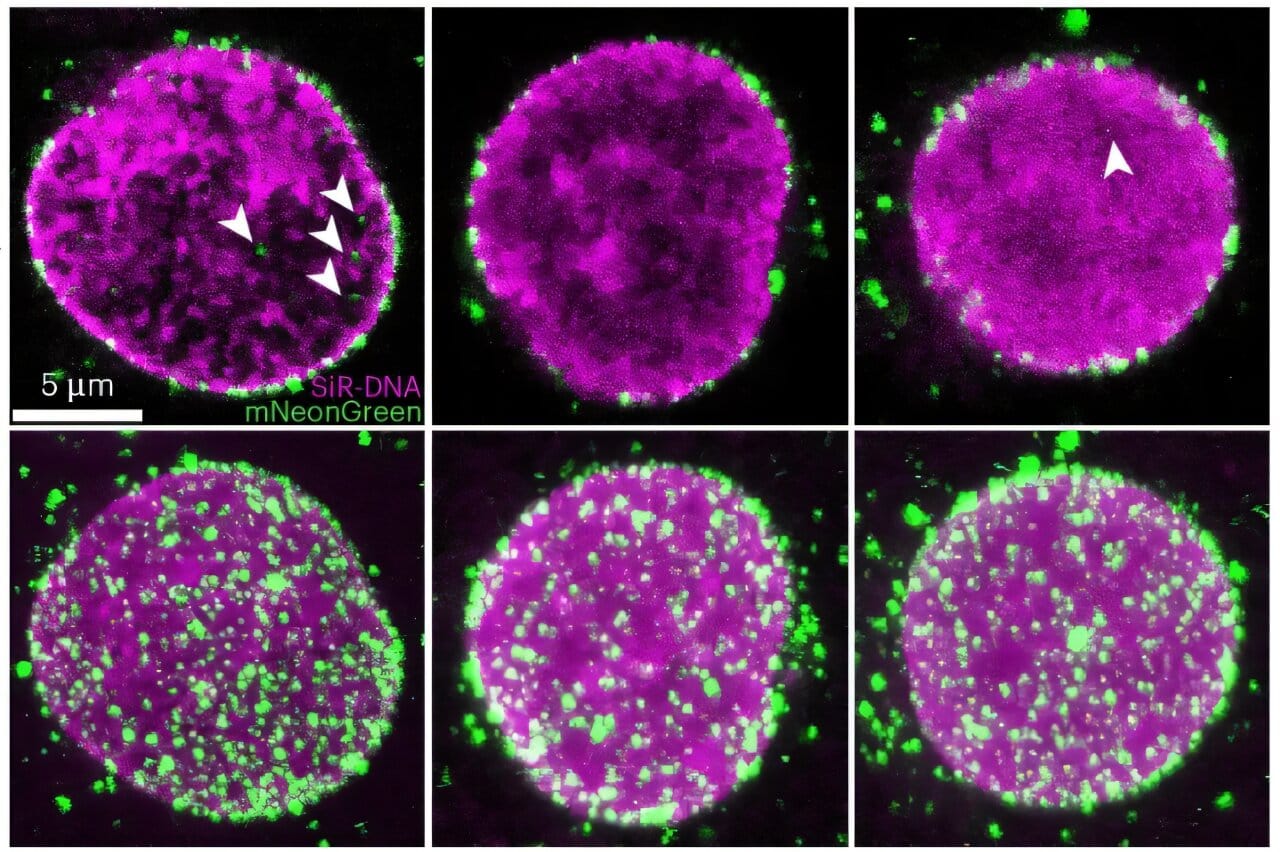
Using this approach, Zhang’s team captured images of nearly 1,500 individual HIV-1 viral cores—the bullet-shaped protein shells that carry the virus’s genetic material—at various stages of entering the nucleus. For the first time, scientists weren’t speculating about how the virus gets in. They were watching it happen.
The findings, published in Nature Microbiology, show that the nuclear pore complex (NPC) is not the fixed, unmoving barrier it was once thought to be. Instead, it’s more like a dynamic, flexible gateway—one capable of reshaping itself in response to certain biological interactions. It adapts its form to allow entry to some molecular guests and deny others, a level of sophistication that redefines our understanding of how cells regulate access to their most intimate structures.
Shape-Shifters at the Gate
HIV-1’s weapon of choice in this covert invasion is its viral core, which packages and protects its RNA genome until the moment it can integrate into the host DNA. The structure of this core, long assumed to disassemble before entering the nucleus, now appears to remain largely intact during import—a stunning reversal of what was once considered biological dogma.
This structural integrity turns out to be critical. The virus must maintain a delicate balance: its core needs to be rigid enough to protect the viral genome and facilitate transport but also flexible enough to maneuver through the tight confines of the nuclear pore. Too brittle, and it falls apart outside the nucleus; too tough, and it can’t make the squeeze.
Even more astonishing is how the nuclear pore responds to this challenge. The once-static gate expands, flexes, and morphs to accommodate the core’s unusual dimensions. It’s a bit like watching a stone archway bend and widen to let a Trojan horse roll through. The nuclear pore is no longer just a doorman. It’s a participant in the viral invasion.
CPSF6: The Inside Man
But the shape of the core and the pliability of the pore aren’t the whole story. The virus needs help from the host—a kind of cellular accomplice. That help comes in the form of CPSF6, a human protein once thought to be a mere bystander in gene expression. Now, it’s emerging as a crucial player in HIV-1’s nuclear ambitions.
CPSF6 binds to the viral core and acts as a molecular escort, guiding it toward and through the nuclear pore. Without this interaction, many viral cores stall at the gate, stuck between the cytoplasm and the nucleus, unable to complete the final leap into the genetic sanctum. The virus, it turns out, is not just sneaking in—it’s being welcomed in by the machinery of the very cells it seeks to destroy.
When the Invasion Fails
Interestingly, not all viral cores succeed. The study revealed that many are filtered out—blocked either by a failure to engage with CPSF6 or because their structural integrity falters under pressure. These defective cores either disintegrate in the cytoplasm or linger uselessly at the nuclear pore’s threshold.
This suggests the nuclear pore plays an active role in quality control. It’s not just opening and closing blindly. It’s evaluating what approaches and deciding—based on interactions and structure—whether to allow entry. In this way, the cell is fighting back, selecting which threats to ignore and which to stop.
This idea fundamentally changes how we view viral infection. Rather than being passive victims of an aggressive intruder, human cells exhibit a nuanced ability to resist, filter, and perhaps even stall infection if the right molecular defenses are engaged.
Why This Matters More Than Ever
HIV-1 remains a global health crisis. Since the first cases were reported in 1981, over 42 million lives have been lost, and more than 38 million people are currently living with HIV/AIDS. Despite enormous advances in treatment and prevention, there is still no cure—and for millions, antiretroviral therapy remains the only line of defense.
That’s why this new understanding is so important.
If scientists can pinpoint exactly how HIV-1’s core navigates into the nucleus—how it maintains its structure, how it engages CPSF6, and how the nuclear pore permits its passage—then it becomes possible to imagine new classes of antiviral drugs. Instead of targeting the virus directly (which often leads to drug resistance), researchers could target the interaction points: prevent CPSF6 from binding to the core, stabilize the nuclear pore to restrict entry, or destabilize the core so it collapses before reaching the nucleus.
These strategies might not just stop HIV-1—they could be broadly applicable to other viruses that exploit similar pathways. From hepatitis B to herpesviruses, understanding how pathogens cross the nuclear threshold may open up entirely new frontiers in infectious disease research.
Seeing the Unseen
More than just a scientific victory, this work highlights the power of in situ structural biology—a field that studies biological molecules in their native environment. Instead of isolating parts of cells and studying them in petri dishes or artificial systems, researchers are now watching biology happen in place, in real time, inside intact cellular architecture.
This is not just zooming in. It’s zooming in with purpose, revealing relationships and processes that were once impossible to observe. It’s the difference between seeing blueprints and walking through the building.
Peijun Zhang’s team didn’t just show what HIV-1 does—they showed how it does it, and when, and where. These temporal and spatial dimensions bring us closer than ever to decoding one of nature’s most sophisticated predators.
The Next Chapter in the Fight
Science is often about small victories—each paper adding a brick to the fortress of knowledge. But every so often, a study comes along that feels like a gate itself has swung open. This is one of those studies.
It reminds us that even the most devastating viruses are subject to the rules of biology, that every invasion has a process, and that processes can be understood—and interrupted.
HIV-1 may be a master of disguise and infiltration, but it’s not infallible. We now know more than ever about how it moves, what it needs, and where it fails. That knowledge is power—the kind of power that could eventually help us disarm the virus once and for all.
As we stand on the shoulders of electron microscopes and molecular imaging, we’re no longer just reacting to the virus—we’re beginning to anticipate it, to counter its every move before it makes it.
And that could be the difference between a life lived with HIV and a world where HIV is no longer a threat at all.
Reference: Zhen Hou et al, HIV-1 nuclear import is selective and depends on both capsid elasticity and nuclear pore adaptability, Nature Microbiology (2025). DOI: 10.1038/s41564-025-02054-z