Cancer treatments often walk a tightrope between healing and harm. In the case of PARP inhibitors, a class of powerful medications used to treat ovarian, breast, and other cancers, that delicate balance has long posed a dilemma for researchers and clinicians. These drugs are designed to wreak havoc on cancer cells by sabotaging their DNA repair systems—but a puzzling and persistent problem has dogged their otherwise promising performance.
While effective against tumor cells, PARP inhibitors have a hidden dark side: they also damage the T cells—the very immune warriors needed to finish the job. This biological backfire has left scientists wondering how a drug meant to mobilize the immune system could end up weakening it, and it may help explain why PARP inhibitors, though widely used, often fall short of delivering complete tumor destruction.
Now, new research from a multi-institutional team of scientists in Wuhan, China, is shedding light on this paradox—and offering a way to fix it. Writing in Science Translational Medicine, the team reports that by blocking the self-sabotaging effects of PARP inhibitors on T cells, they were able to amplify the drugs’ power and unlock new potential for immunotherapy combinations. The findings could expand the use of PARP inhibitors and reinvigorate stalled cancer treatments with life-saving implications for patients.
What Are PARP Inhibitors—and Why Do They Hurt T Cells?
To understand the problem, it’s important to know what PARP inhibitors are designed to do. These drugs target an enzyme called poly (ADP-ribose) polymerase, or PARP, which plays a critical role in helping cells repair their DNA. Cancer cells often rely heavily on this repair pathway, especially when their other defenses are compromised, such as in tumors with BRCA gene mutations.
By blocking PARP, the drugs essentially push already vulnerable cancer cells over the edge, making it harder for them to survive DNA damage caused by the treatment. This is a well-established therapeutic strategy, and it’s part of why seven different PARP inhibitors have been approved globally to treat ovarian, breast, prostate, and pancreatic cancers.
However, as Dr. Jiahao Liu and his colleagues from Tongji Hospital, Tongji Medical College, and Huazhong University of Science and Technology discovered, these medications come with an unintended side effect: they also harm the immune system’s T cells.
T cells are the body’s first line of defense against cancer. They seek out malignant cells and destroy them—especially when helped along by therapies like CAR T-cell therapy or immune checkpoint inhibitors. But in patients treated with PARP inhibitors, these immune cells showed signs of DNA damage, slower replication, and higher rates of programmed cell death (apoptosis).
This is more than just collateral damage—it’s a self-defeating cycle. A drug designed to empower the immune system ends up crippling it, limiting the treatment’s success and potentially allowing tumors to escape destruction.
Tracking the Damage: What the New Research Shows
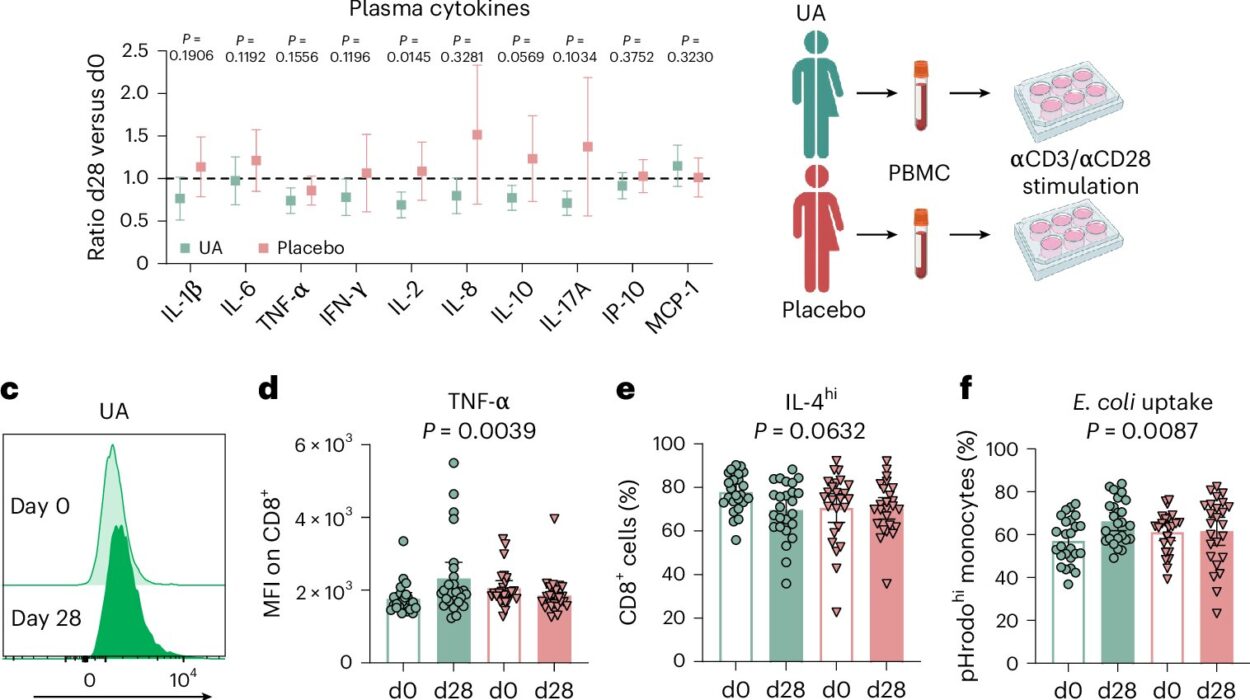
To get to the bottom of this problem, Liu’s team used human tissue samples and mouse models to study what happens to T cells during PARP inhibitor treatment. They found that even though the drugs were attacking tumor cells as expected, they were also damaging the DNA of healthy T cells, reducing their numbers and making them less effective.
The key culprit? A protein known as PARP1, which gets trapped on DNA when the inhibitors are at work. This “PARP1 trapping” is what actually causes the DNA repair machinery in tumor cells to break down—but it also disrupts normal functioning in T cells.
In other words, the very mechanism that makes PARP inhibitors effective is also what makes them toxic to T cells.
Using CRISPR gene-editing, the researchers were able to delete the PARP1 gene in T cells or mutate its binding sites. This genetic tweak prevented DNA damage and protected T cells from harm, while also improving the overall efficacy of the treatment in lab mice. When they combined PARP inhibitors with checkpoint inhibitors like olaparib, the results were even more promising.
Engineering Stronger CAR T Cells
Perhaps the most groundbreaking part of the study was what happened when researchers engineered new versions of CAR T cells—a form of genetically modified immune cells that can be trained to attack specific cancers. These new CAR T cells were designed to resist the effects of PARP inhibitors.
The modified T cells not only survived the treatment, but they also performed better, eradicating ovarian tumors more effectively than standard CAR T cells. By reducing PARP1 trapping, the researchers could dial down the DNA damage in immune cells while keeping up the pressure on cancer.
This dual benefit opens the door for a new era of combination therapies, where drugs and immune cells can work in harmony rather than at cross purposes.
A Cure Within Reach—or Another Roadblock?
There is still a long way to go. The study’s authors acknowledge a scientific paradox that continues to puzzle researchers: While PARP inhibitors can damage T cells, they also seem to stimulate immune responses under certain conditions. Some studies suggest that these drugs can actually help activate the immune system, potentially making it easier to eliminate cancer cells.
This contradiction has made it difficult to predict when and how PARP inhibitors will be most effective. It also helps explain why combinations with immunotherapy have produced inconsistent results in clinical trials. But the new research provides a powerful clue: By minimizing T cell DNA damage, doctors might be able to control the double-edged effects of PARP inhibitors and harness their full power.
“This study highlights the relevance of PARP inhibitor-induced DNA damage to T cells and suggests opportunities to improve the efficacy of PARP inhibitors as monotherapy or in combination with immunotherapy,” Liu and his colleagues concluded.
Implications for the Future of Cancer Treatment
What does this mean for patients?
If these findings hold true in clinical settings, oncologists may soon be able to refine cancer treatments that were previously blunt instruments. By protecting the immune system from friendly fire, doctors could extend the life-saving potential of PARP inhibitors and bring renewed hope to people fighting some of the most aggressive cancers.
There’s also the tantalizing possibility that existing PARP inhibitors could be redesigned to avoid harming T cells or that patients could receive companion treatments that shield their immune systems during therapy. In either case, the study brings urgently needed clarity to a field long clouded by contradiction.
From the Lab to the Clinic: What Comes Next?
The road ahead will include larger animal studies and eventual human clinical trials to determine whether the results seen in the lab translate into better outcomes for real patients. Researchers will also have to test the long-term safety of engineered CAR T cells and explore how these discoveries might apply to other cancers treated with PARP inhibitors.
Still, the breakthrough represents a significant step forward. By identifying a key weakness in a cornerstone cancer treatment and showing how to fix it, the Wuhan team has moved the field closer to the goal of more targeted, personalized, and effective cancer therapies.
For now, PARP inhibitors remain powerful but imperfect tools. Thanks to new discoveries like these, the future may hold a version of these drugs that not only crushes cancer cells but also arms the immune system to keep fighting long after the treatment ends.
In the war against cancer, it turns out that sometimes the hardest battle is learning not to hurt your own soldiers. And that, perhaps, is where victory begins.
More information: Jiahao Liu et al, Mitigating T cell DNA damage during PARP inhibitor treatment enhances antitumor efficacy, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adr5861