Throughout the history of life on Earth, an invisible war has raged. It is a battle fought not on distant fields or within national borders, but within bodies—ours, and those of nearly every living organism. The combatants are stealthy, adaptable, and relentless. They are viruses, bacteria, fungi, and protozoa—the pathogens that cause disease. And while our immune systems are formidable defenders, they are not alone. Medicine, hygiene, vaccines, and public health measures have shaped the battlefield. Yet even as we innovate, our microbial adversaries adapt. Pathogens evolve. And their evolution is one of the most consequential biological phenomena of our time.
From the mutating influenza virus to the enduring menace of tuberculosis, the evolution of disease is not just a curiosity—it is a central narrative in the story of humanity. It determines how long we live, how societies flourish or fall, and how prepared we are for the next global health crisis.
To understand how diseases change over time is to glimpse the ceaseless evolutionary struggle that shapes both pathogens and their hosts. This story isn’t merely about survival—it’s about strategy, genetics, and the unpredictable dance between predator and prey, parasite and host, microbe and civilization.
Pathogens and the Mechanics of Evolution
To understand how disease evolves, we must first revisit the basic principles of evolution. At its core, evolution is change over generations in the genetic composition of a population. When that population is a virus or bacterium, evolution can occur at breathtaking speed.
Microorganisms replicate rapidly. A single bacterium might divide every twenty minutes under ideal conditions. A virus like influenza or HIV can churn out billions of copies within a single infected host. And in each replication event lies the potential for mutation—random changes in genetic material that may confer advantages or disadvantages.
Most mutations are harmless or even detrimental to the pathogen. But occasionally, a mutation improves a microbe’s ability to infect, replicate, evade immune responses, or resist treatment. Natural selection, the engine of evolution, favors these rare changes. Over time, they accumulate, and new strains emerge—sometimes more dangerous, sometimes more transmissible, sometimes more resistant to medicine.
Horizontal gene transfer adds another twist. Unlike humans and most animals, many microbes can exchange genetic material directly. Bacteria, for instance, can share genes through plasmids—circular bits of DNA that often carry resistance traits. This microbial generosity accelerates evolution, especially when it comes to surviving antibiotics or adapting to new hosts.
The Dance of Coevolution
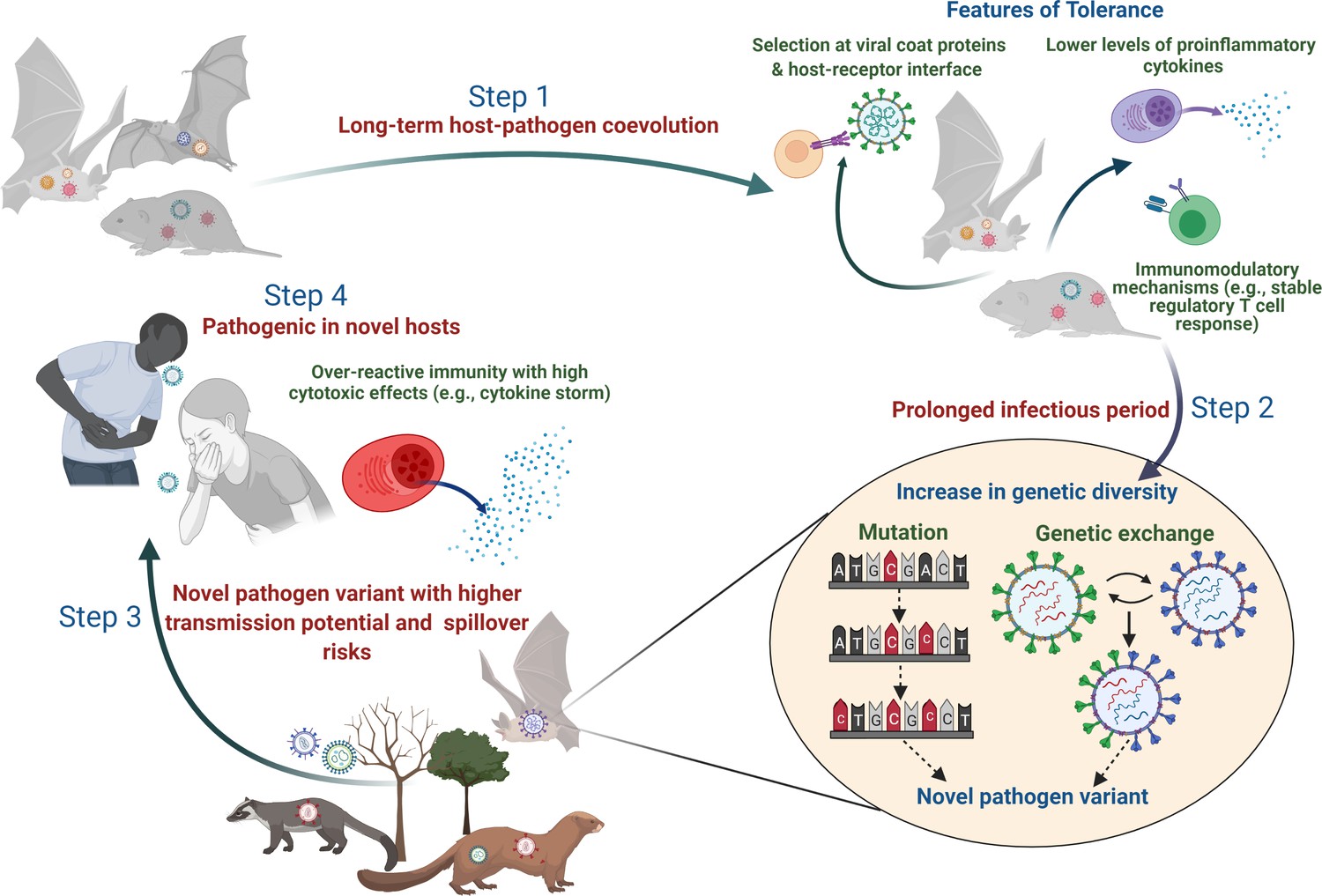
Pathogens don’t evolve in a vacuum. They evolve alongside their hosts—an evolutionary waltz known as coevolution. This reciprocal process means that as we adapt defenses, pathogens refine their offenses.
Consider the immune system, one of the most sophisticated defense mechanisms in the natural world. It recognizes molecular patterns on pathogens and launches targeted attacks. Yet pathogens strike back. Viruses like HIV can mutate their surface proteins so rapidly that the immune system is always a step behind. Some bacteria, like those responsible for gonorrhea, use antigenic variation to alter their outer membranes, effectively wearing different disguises each time they invade.
Vaccines, too, exert evolutionary pressure. They prime the immune system against specific pathogens, reducing their prevalence and breaking chains of transmission. But occasionally, a mutation arises that allows the pathogen to escape immune detection—creating what scientists call “vaccine escape variants.” This is a rare but real evolutionary pathway, especially when vaccination coverage is patchy or the pathogen has high mutation rates.
Human evolution also reflects this coevolutionary struggle. Certain genetic traits—like sickle cell anemia—persist because they confer resistance to diseases like malaria. Our genomes bear the scars of ancient pandemics, with remnants of viral DNA embedded in our chromosomes and genes shaped by millennia of microbial war.
Influenza: A Masterclass in Adaptation
Of all the evolving pathogens, influenza may be the most instructive. It is the ultimate shapeshifter, a virus that reinvents itself regularly. This is partly why flu vaccines are updated every year—each season brings a different mix of strains.
Influenza evolves through two main processes: antigenic drift and antigenic shift. Drift involves gradual mutations in the virus’s surface proteins—hemagglutinin (H) and neuraminidase (N). These small changes accumulate over time, allowing the virus to dodge immune memory. This is why you can catch the flu multiple times in your life, even if you’ve had it before.
Antigenic shift, on the other hand, is far more dramatic. It happens when two different influenza viruses infect the same host—often a pig or bird—and swap genetic segments. The result is a hybrid virus with novel surface proteins. If such a virus can infect humans and spread easily, it can spark a pandemic. The 1918 Spanish flu, which killed tens of millions, was one such event. So was the 2009 H1N1 pandemic.
Influenza’s ability to evolve isn’t just a biological curiosity—it’s a public health challenge. The virus is a moving target, and its evolution demands constant surveillance, global cooperation, and rapid vaccine development. In many ways, influenza is a mirror reflecting our vulnerability to nature’s smallest predators.
Antibiotic Resistance: Evolution’s Dark Gift
Perhaps the most pressing example of microbial evolution is antibiotic resistance. When Alexander Fleming discovered penicillin in 1928, it seemed like the dawn of a new era. Infections that had once been fatal—strep throat, pneumonia, syphilis—were now curable. But even then, Fleming warned that misuse of antibiotics could lead to resistance.
He was right. The more we use antibiotics, the more we select for resistant strains. Bacteria that survive antibiotic exposure pass on their resistant genes. Over time, entire populations become impervious to drugs that once wiped them out.
This isn’t hypothetical—it’s happening now. Multi-drug-resistant tuberculosis (MDR-TB), methicillin-resistant Staphylococcus aureus (MRSA), and carbapenem-resistant Enterobacteriaceae (CRE) are not just names in journals; they are deadly realities in hospitals around the world.
Some bacteria have evolved enzymes like beta-lactamases that dismantle penicillin-like drugs. Others change their cellular targets so that antibiotics no longer bind effectively. Still others pump drugs out of their cells before they can do harm.
And resistance doesn’t stay in one species. Through plasmids and mobile genetic elements, resistance can spread from harmless bacteria to deadly ones—a microbial version of espionage. It is evolution at its most cunning, and our overuse of antibiotics in medicine and agriculture has only hastened it.
The World Health Organization now considers antimicrobial resistance one of the top ten global health threats. If we continue on our current path, we could enter a “post-antibiotic era” where routine infections become deadly once more.
Pandemics Past and Future
The history of human civilization is interwoven with the history of pandemics. The Black Death in the 14th century, caused by Yersinia pestis, reshaped the demographics of Europe. Smallpox devastated indigenous populations in the Americas. Cholera swept across continents. HIV/AIDS emerged in the 20th century and continues to affect millions. And most recently, SARS-CoV-2—responsible for COVID-19—changed the world in ways still unfolding.
Each of these pathogens evolved in response to ecological conditions, host availability, and the selective pressures of human society. Urbanization, travel, deforestation, and climate change have all increased the opportunities for disease emergence.
Zoonotic spillovers—where pathogens jump from animals to humans—are often the flashpoint. SARS, MERS, Ebola, and COVID-19 all originated this way. The pathogens responsible were not “new” in nature, but newly adapted to humans. Their genetic changes, often involving mutations that improved binding to human cell receptors, allowed them to spread efficiently once the jump was made.
Viruses are especially adept at this. Their small genomes and rapid replication mean that even within a single host, thousands of variants may arise. Natural selection filters them, favoring the ones best suited for human transmission. The result is a pathogen perfectly tailored for its new host.
Predicting the next pandemic isn’t easy, but understanding how pathogens evolve improves our odds. Genomic surveillance, rapid diagnostics, and evolutionary modeling are tools we now wield to stay one step ahead.
Vaccines and the Evolutionary Battlefield
Vaccines are among the most powerful tools in our medical arsenal. They train the immune system to recognize and neutralize pathogens, often before infection can take hold. But they also exert evolutionary pressure.
When a vaccine targets a specific part of a virus—like the spike protein of SARS-CoV-2—mutations in that region can lead to “escape variants.” This happened with the emergence of variants like Delta and Omicron. Though vaccines still protected against severe disease, the virus’s evolution demanded updates and boosters.
Pathogens evolve differently depending on the type of immunity they face. Natural immunity, which may be broad but inconsistent, exerts different pressures than vaccine-induced immunity, which tends to be more specific. In some cases, vaccines can drive a pathogen to evolve lower virulence—after all, killing the host quickly is a poor transmission strategy. In other cases, particularly when immunity is incomplete or uneven across a population, evolution may favor higher transmissibility.
The design of vaccines matters, too. “Leaky” vaccines, which reduce symptoms but don’t fully block transmission, can allow virulent strains to circulate silently. On the other hand, sterilizing immunity—where the vaccine prevents infection entirely—can halt evolution in its tracks.
The ongoing challenge is to develop vaccines that are both effective and evolution-resistant. Universal vaccines, like those being explored for influenza and coronavirus, aim to target conserved regions of the virus—areas less prone to mutation. If successful, they could reduce the evolutionary escape routes available to pathogens.
The Role of Environment and Society
Pathogen evolution does not occur in a biological vacuum. It is influenced—often dramatically—by human behavior and environment.
Crowded urban settings, poor sanitation, and lack of healthcare infrastructure provide fertile ground for disease evolution. Pathogens that thrive in such conditions often become more transmissible or more persistent.
Global travel means a mutation in a remote village can reach a megacity in a matter of hours. Climate change alters the range of disease vectors like mosquitoes, introducing diseases like dengue and Zika to new regions. Deforestation brings humans into contact with wildlife reservoirs, increasing the risk of spillover.
Agricultural practices, too, play a role. The overuse of antibiotics in livestock, the monoculture of plants, and intensive farming all create ecosystems where pathogens can evolve rapidly. In some cases, animals act as mixing vessels—like pigs with flu—where human and animal viruses combine.
Human culture, economics, and politics all shape how diseases evolve and spread. War, displacement, poverty, and mistrust of science can undo decades of public health gains in an instant.
Toward an Evolutionary Medicine
Understanding the evolution of disease is not just a scientific endeavor—it is a public health imperative. The emerging field of evolutionary medicine seeks to integrate evolutionary biology into clinical practice. It asks not just how to treat disease, but why disease exists in its current form.
Why do some viruses become less deadly over time, while others grow more virulent? How do treatment regimens influence microbial evolution? What social policies can slow the rise of resistance?
These are not idle questions. They are strategies for survival in an age where microbes continue to evolve faster than we do. Evolutionary thinking can inform how we use antibiotics, design vaccines, manage hospital environments, and prepare for the next outbreak.
Even cancer is now being understood through an evolutionary lens—as a population of rogue cells adapting within the ecosystem of the body. This shift in perspective opens up new avenues for treatment and prevention.
The Never-Ending Story
There will never be a final victory in the war against disease. Pathogens will continue to evolve as long as life persists. But this is not cause for despair. It is a call to be smarter, more adaptive, and more humble.
We are not passive victims in this story. Our knowledge grows. Our tools improve. Our understanding of evolution gives us foresight. We can anticipate resistance. We can outmaneuver new variants. We can build systems that are resilient, flexible, and informed by the deep logic of life’s constant change.
In this evolutionary arms race, we may never stop running—but we can learn to run faster, with more insight and intention.
Pathogens evolve. But so do we.