Every so often, a scientific breakthrough redefines not only what is possible but what it means to be human. Fire gave us control over nature. The printing press democratized knowledge. Antibiotics redefined medicine. The internet restructured global society.
Now, we stand at the precipice of another such revolution—one that takes place at the molecular level, deep within the double helix of DNA.
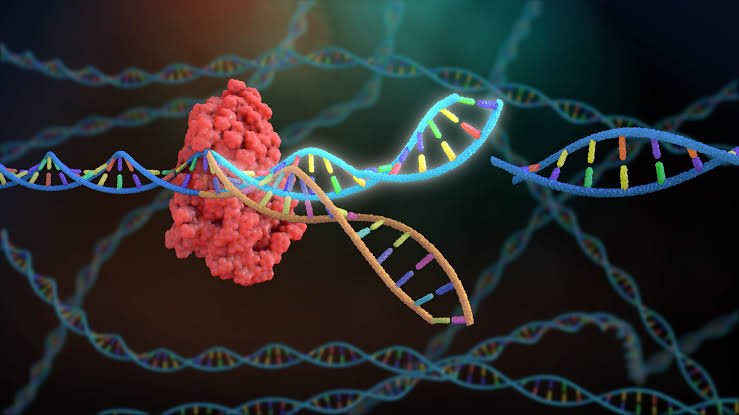
CRISPR is not just a tool. It’s not merely a laboratory technique or the latest acronym tossed about in scientific journals. CRISPR is the key to mastering our genetic code—a technology so powerful that it is reshaping biology, medicine, and even philosophy itself. With it, we are gaining the ability to rewrite the code of life.
CRISPR, which stands for Clustered Regularly Interspaced Short Palindromic Repeats, is a gene-editing technology derived from the natural defense systems of bacteria. In a few short years, it has gone from an obscure area of microbial genetics to one of the most transformative tools in science, capable of editing genes with unprecedented precision, speed, and simplicity.
In this article, we will explore how CRISPR works, what makes it so revolutionary, and how it is transforming the field of genetic medicine—from curing inherited diseases and fighting cancer to possibly engineering immunity, reversing aging, and even shaping the future of evolution.
From Bacterial Defense to Biological Engineering
To understand how CRISPR is revolutionizing medicine, we must first understand where it came from. Ironically, this technology—now at the bleeding edge of human science—originated in one of Earth’s oldest organisms: bacteria.
Bacteria, constantly under attack from viruses called phages, evolved a kind of molecular memory system. When a virus invades, the bacterium chops up its DNA and stores pieces of it within its own genome in clusters—the “CRISPR” regions. These serve as a genetic mugshot of past invaders.
When that virus attacks again, the bacterium transcribes these CRISPR sequences into RNA, which pairs with a protein called Cas9 (CRISPR-associated protein 9). Together, they act like a guided missile system, hunting down matching viral DNA and slicing it apart.
In 2012, Jennifer Doudna and Emmanuelle Charpentier realized that this bacterial defense mechanism could be reprogrammed. By changing the guide RNA, scientists could target any DNA sequence they wanted—effectively creating a gene-editing scalpel that could cut DNA with incredible precision.
The implications were immediate and staggering.
Why CRISPR Is Different: The Scalpel vs. The Sledgehammer
Before CRISPR, gene editing was more like surgery with a sledgehammer than a scalpel. Techniques like zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) allowed for DNA editing, but were expensive, difficult to program, and prone to off-target effects.
CRISPR changed everything.
Here’s what makes CRISPR revolutionary:
- Precision: CRISPR can target specific genes or even single base pairs in the genome.
- Efficiency: Edits can be made quickly and cheaply in almost any organism.
- Accessibility: Labs around the world can now do gene editing without needing massive infrastructure.
- Versatility: CRISPR can delete genes, insert new ones, repair mutations, or turn genes on or off.
This suite of capabilities makes CRISPR the most powerful genetic tool humans have ever wielded. And medicine was one of the first places it began to make waves.
Correcting the Code: CRISPR in Genetic Disease
There are over 6,000 known genetic diseases—caused by everything from a single misspelled DNA letter to massive deletions or duplications in the genome. Many of these conditions are devastating and currently untreatable. Cystic fibrosis. Huntington’s disease. Tay-Sachs. Muscular dystrophy. Sickle cell anemia.
CRISPR offers hope to millions.
By correcting faulty genes at the source, CRISPR moves beyond symptom management to actual cures.
Take sickle cell disease, for example. This painful and life-shortening condition is caused by a single mutation in the gene for hemoglobin. In 2019, a woman named Victoria Gray became the first patient in the U.S. to receive CRISPR therapy for sickle cell disease. Doctors edited her bone marrow cells to produce a fetal form of hemoglobin that doesn’t sickle. The results? Dramatic improvement. No more crises. No more hospitalizations.
Other clinical trials are underway for beta-thalassemia, a similar blood disorder. Scientists are also developing CRISPR treatments for inherited blindness (like Leber congenital amaurosis), Duchenne muscular dystrophy, and even rare forms of epilepsy.
In some cases, the strategy is ex vivo—scientists remove cells, edit them in the lab, then reinfuse them. In others, they use in vivo approaches, delivering CRISPR directly into the body using viral vectors or lipid nanoparticles.
Either way, the promise is the same: not just treating genetic disease—but erasing it.
CRISPR vs. Cancer: Rewriting Fate
Cancer is, at its core, a disease of genetic mutation. Tumor cells mutate uncontrollably, evading immune surveillance, hijacking growth pathways, and spreading across the body.
CRISPR provides a powerful new weapon in the fight.
One major use is in engineering CAR-T cells—immune cells modified to better recognize and kill cancer. Using CRISPR, scientists can knock out genes that tumors use to evade detection or suppress immune response. They can also introduce genes that make T cells more potent.
In 2020, researchers launched the first U.S. trial using CRISPR to treat cancer patients with engineered immune cells. The early results were promising: edited T cells were safe, persistent, and showed signs of attacking the cancer.
Another approach is using CRISPR to identify cancer vulnerabilities. By systematically turning off genes across thousands of tumor cells, researchers can find which genes are essential for cancer survival—and then target them with drugs or further gene editing.
Some labs are even exploring CRISPR gene drives that could target cancer-causing viruses like HPV or Epstein-Barr inside infected cells.
Cancer is cunning, but CRISPR gives doctors a new level of control—a way to rewrite fate at the genetic level.
Infectious Diseases: Editing Our Immune Defense
CRISPR’s ability to edit genes isn’t limited to inherited mutations. It can also be used to enhance immunity, combat viruses, and control pathogens.
During the COVID-19 pandemic, researchers used CRISPR not only to develop rapid diagnostic tests but also explored CRISPR-based antivirals. One system, called PAC-MAN (Prophylactic Antiviral CRISPR in huMAN cells), used Cas13 to target and degrade coronavirus RNA inside cells.
In the fight against HIV, CRISPR offers hope for a cure. By targeting and cutting out viral DNA embedded in human cells, scientists have already demonstrated complete viral eradication in mice. Human trials are underway.
Some researchers are using CRISPR to create disease-resistant mosquitoes to combat malaria, or to engineer pigs free from viruses that could jump to humans during organ transplants.
Whether through diagnostics, vaccines, or immune enhancement, CRISPR is becoming a crucial tool in the war on infectious disease.
CRISPR and Rare Diseases: Precision for the Few
One of the most exciting applications of CRISPR is in treating ultra-rare diseases—conditions so uncommon they affect fewer than 1 in a million people.
Traditionally, drug companies ignored these diseases because treatments weren’t profitable. CRISPR changes that. Its flexibility allows for the rapid design of therapies tailored to an individual’s mutation.
In 2020, a young girl named Victoria was treated for CLN7 Batten disease, a rare neurodegenerative disorder, using a CRISPR-inspired therapy designed specifically for her. Dubbed “N-of-1” medicine, this approach represents a new paradigm—personalized genetic therapies for each patient.
As costs fall and technology improves, what was once unthinkable—custom genetic medicine—is becoming reality.
Beyond the Body: CRISPR in Organoids and Gene Therapy
CRISPR isn’t just fixing genes in the body. It’s revolutionizing how we study and understand disease.
Scientists now use CRISPR to create organoids—tiny, lab-grown versions of human organs like brains, livers, and intestines. These models can be used to study disease progression, test drugs, and simulate genetic disorders.
CRISPR also plays a vital role in gene therapy, allowing for the safe integration of therapeutic genes into the genome. Unlike older techniques, which randomly inserted genes and risked causing cancer, CRISPR enables precise, site-specific edits.
This precision may allow us to treat conditions like hemophilia, severe combined immunodeficiency (SCID), and even metabolic disorders like phenylketonuria.
Ethical Frontiers: The Genome in Our Hands
With great power comes great responsibility.
CRISPR’s ability to edit the human genome raises profound ethical questions. Should we use it to eliminate inherited disease? Most say yes. But what about editing embryos? Enhancing intelligence? Creating “designer babies”?
In 2018, Chinese scientist He Jiankui stunned the world by announcing he had edited the genomes of twin girls to make them resistant to HIV. The global scientific community condemned the move as reckless, premature, and unethical. He was imprisoned. But the genie was out of the bottle.
The question isn’t if we can edit embryos. It’s whether we should—and under what conditions.
Bioethicists, lawmakers, and scientists are now scrambling to develop international guidelines for human germline editing. Most agree that therapeutic editing—to prevent deadly diseases—should be considered separately from enhancement or aesthetic modification.
But the lines are blurry. What about increasing resistance to Alzheimer’s? Or improving memory, muscle growth, or immunity?
These debates will define the next century of medicine. CRISPR doesn’t just let us edit DNA—it forces us to redefine what it means to be human.
The Future: Gene Writing, Not Just Editing
Even as CRISPR transforms medicine today, new iterations are already expanding its possibilities.
- Base editing allows scientists to change a single DNA letter without cutting the strand—ideal for diseases caused by point mutations.
- Prime editing is a newer technique that acts like a DNA word processor, capable of searching and replacing long sequences with high accuracy.
- Epigenome editing can modify gene expression without altering the underlying DNA, potentially reversing conditions like obesity or diabetes.
Researchers are also developing RNA-targeting CRISPRs, which could treat viral infections or neurological diseases like ALS by regulating gene expression in real-time.
The ultimate vision is programmable biology: writing and rewriting our biology as easily as we program computers today.
A Final Word: Power, Promise, and Perspective
CRISPR is not magic. It’s science at its most potent—an elegant system borrowed from nature, harnessed by human ingenuity.
It is already saving lives, rewriting destinies, and redefining what’s possible in medicine. From inherited diseases to cancer, from pandemics to organ transplantation, CRISPR is leading a revolution—not tomorrow, but today.
But with this power comes responsibility. We must tread carefully, humbly, and wisely. The human genome is not a toy or a spreadsheet—it is the sacred blueprint of life. And as we learn to edit that blueprint, we must ensure our goals are guided not just by what we can do, but what we should do.
The age of CRISPR has begun. The rest is up to us.