Imagine a future where damaged hearts repair themselves after a heart attack. Where spinal cord injuries no longer mean paralysis. Where diabetes, Parkinson’s, and even some forms of blindness can be cured—not with pills or surgery—but by regrowing the affected tissues from scratch. It sounds like science fiction, but this is the bold promise of stem cell medicine.
Stem cells, with their remarkable ability to become many different cell types, have captured the imaginations of scientists, doctors, and patients alike. They’re often hailed as the holy grail of regenerative medicine. But while their potential is vast, the road from lab bench to hospital bedside is long, winding, and filled with both promise and pitfalls.
In this deep dive, we’ll explore the science behind stem cells, the real medical breakthroughs already underway, the risks and limitations, and the ethical and regulatory challenges that shape this evolving field. This is the story of stem cells: where hope meets hype—and how science is working to separate the two.
What Are Stem Cells?
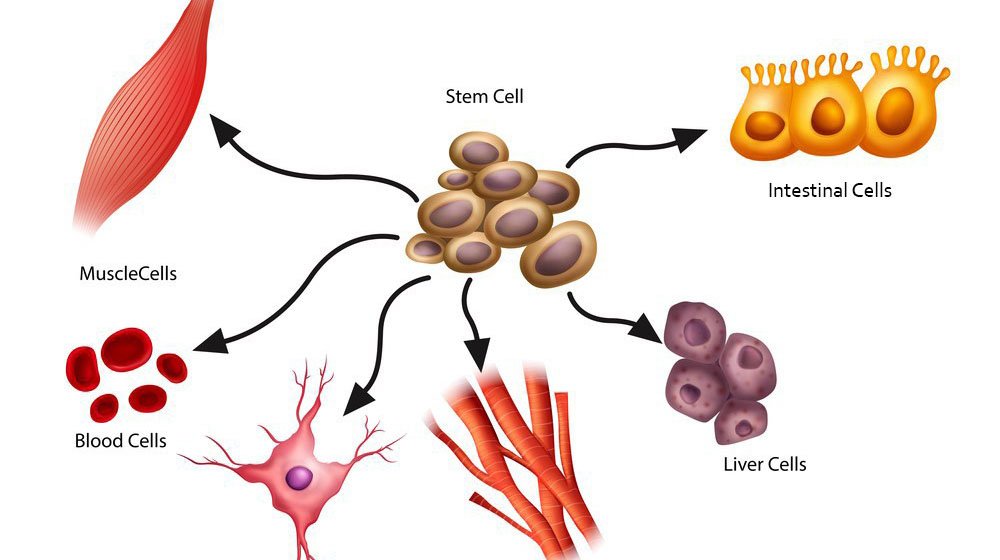
At the most basic level, stem cells are unspecialized cells that can develop into many different cell types in the body. They serve as a kind of repair system, with the ability to divide almost indefinitely and to replenish other cells.
There are two main characteristics that define stem cells:
- Self-renewal – They can divide and produce more stem cells.
- Potency – They can differentiate into specialized cells, such as muscle, nerve, or blood cells.
Stem cells fall into several categories based on their source and potential:
Embryonic Stem Cells
These are the most potent stem cells, harvested from early-stage embryos (typically 3–5 days after fertilization). They are pluripotent, meaning they can become any cell type in the body. However, their use raises significant ethical concerns because it involves the destruction of human embryos.
Adult Stem Cells
These exist in various tissues of the adult body, such as bone marrow or fat. They are multipotent—they can give rise to several related cell types, but not all. For example, hematopoietic stem cells from bone marrow can become different types of blood cells, but not neurons or liver cells.
Induced Pluripotent Stem Cells (iPSCs)
Discovered in 2006, iPSCs are adult cells (often skin or blood cells) that have been genetically reprogrammed to return to an embryonic-like state. These cells behave like embryonic stem cells but don’t require embryos, sidestepping many ethical issues. iPSCs have opened vast new avenues for research and therapy.
The Science of Regeneration
What makes stem cells so powerful is not just their ability to replicate, but their ability to differentiate. Scientists can coax stem cells into becoming nearly any tissue in the body. This opens up incredible possibilities for regenerative medicine, where damaged tissues or organs could be repaired or replaced.
Here’s how this process might work:
- Isolation: Stem cells are harvested from the patient (autologous) or a donor (allogeneic).
- Cultivation: In the lab, scientists grow and expand the stem cells.
- Differentiation: Using chemical signals and growth factors, the stem cells are guided to become specific types of cells—heart muscle, neurons, cartilage, etc.
- Transplantation: The newly grown cells are implanted back into the patient to repair or regenerate damaged tissues.
This process is already being tested in numerous clinical trials and has shown promise in areas ranging from blood diseases to spinal injuries.
Stem Cell Therapies Today: Real Progress
Despite the futuristic aura, stem cell therapies are not all hype. Some have been successfully used for decades, while others are on the cusp of broader medical approval.
Bone Marrow Transplants
The earliest and most successful stem cell therapy is the bone marrow transplant, used to treat leukemia and other blood disorders. Here, hematopoietic stem cells (HSCs) from a donor or the patient are transplanted to regenerate a healthy blood system after chemotherapy or radiation.
Skin Grafts and Burns
Stem cells have been used to regenerate skin in burn victims. Lab-grown sheets of skin using stem cells have saved lives and are now a routine part of severe burn treatment in advanced medical centers.
Eye Conditions
Corneal stem cell transplants have restored vision in people with certain kinds of blindness caused by burns or injury. Trials are also exploring stem cell treatments for macular degeneration, a leading cause of blindness in older adults.
Cartilage and Joint Repair
Early trials have used mesenchymal stem cells (MSCs) to regenerate cartilage in joints affected by arthritis. Though results are still mixed, there is cautious optimism that stem cells could one day delay or replace the need for joint replacement surgeries.
Type 1 Diabetes
One of the most promising areas of stem cell therapy is the development of insulin-producing beta cells for diabetics. Companies like Vertex Pharmaceuticals are testing stem cell-derived islet transplants to restore the body’s natural insulin regulation.
On the Horizon: Emerging Applications
As research progresses, the list of conditions potentially treatable by stem cells grows longer. These applications remain experimental, but they could redefine medicine in the decades to come.
Parkinson’s Disease
By replacing lost dopamine-producing neurons, stem cell therapy could offer a functional cure for Parkinson’s. Several clinical trials are underway to test the safety and efficacy of these implants.
Spinal Cord Injuries
Patients with spinal cord damage have few options today. Stem cell implants might help restore some motor function or sensory control. Early trials have shown encouraging—though modest—improvements in select patients.
Heart Disease
Cardiomyocytes derived from stem cells are being tested as treatments for heart attack patients. The idea is to regenerate damaged heart muscle and improve cardiac function—something no drug can currently do.
Liver and Lung Diseases
Organoids—miniature, simplified organs grown from stem cells—could pave the way for lab-grown replacements for damaged liver or lung tissue. This is a long-term goal, but one that’s inching closer with each passing year.
Immunotherapy and Cancer
Stem cells may also have a future in personalized immunotherapy. By engineering immune cells from stem cells, doctors may be able to create highly targeted cancer treatments—tailored to each patient’s tumor.
The Hype: Snake Oil and Stem Cell Scams
With all the excitement surrounding stem cells, it’s not surprising that some people want to cash in on the hype. Unfortunately, this has led to a proliferation of unregulated stem cell clinics around the world—especially in countries with looser medical regulations.
These clinics often promise miraculous cures for everything from autism to Alzheimer’s to chronic pain. They use vague language, cherry-picked anecdotes, and high-pressure sales tactics. Some even charge tens of thousands of dollars for “treatments” that are unproven, unregulated, and potentially dangerous.
There have been cases of:
- Blindness caused by stem cell injections into the eyes
- Tumors growing from improperly differentiated stem cells
- Infections from poorly handled cell cultures
This “stem cell tourism” industry not only harms patients but undermines legitimate scientific progress. The truth is: real science takes time. Breakthroughs in the lab can take years—or decades—to translate into safe, effective therapies.
Ethical Dilemmas and Moral Questions
Stem cell research has sparked intense debate, especially over the use of embryonic stem cells. Opponents argue that harvesting these cells involves destroying human embryos and is therefore morally unacceptable. Proponents argue that these embryos are typically left over from IVF treatments and would be discarded anyway—and that their use can lead to life-saving therapies.
In recent years, the development of iPSCs has helped ease some of this controversy. Because iPSCs do not involve embryos, they sidestep many ethical issues while offering nearly the same research potential.
However, new ethical concerns continue to arise:
- Human cloning: Could iPSC technology be used to create human clones?
- Designer babies: Could stem cell editing be used to enhance traits before birth?
- Chimeras: Researchers have begun inserting human stem cells into animal embryos to grow human-like organs—raising questions about the moral status of these hybrid creatures.
Balancing scientific progress with ethical boundaries is one of the great challenges of our time. It requires open dialogue, regulation, and a willingness to confront difficult questions head-on.
The Role of Regulation and Oversight
In the U.S., stem cell therapies are regulated by the Food and Drug Administration (FDA). Only a few stem cell-based products have received full approval, and most remain under investigation or are available only through clinical trials.
Other countries have their own regulatory systems, but enforcement varies widely. This is one reason why international collaboration is crucial. Stem cell research and therapy are global enterprises, and consistent standards are essential to ensure safety and scientific integrity.
Institutions like the International Society for Stem Cell Research (ISSCR) provide guidelines and frameworks to help scientists, clinicians, and policymakers navigate this complex terrain.
Public Perception and Media Influence
How the public views stem cells is shaped largely by the media—which often oscillates between unrealistic hype and overly cautious skepticism.
One week, a news outlet might proclaim a “stem cell cure” for a disease. The next, it might report a lawsuit over fraudulent treatments. This back-and-forth can confuse the public and erode trust in science.
It’s important for journalists, researchers, and clinicians to communicate clearly. Not every breakthrough is a cure. Not every therapy is ready for prime time. But that doesn’t mean the science isn’t real or important. Nuance is key.
The Future of Personalized Stem Cell Medicine
As the field matures, we are moving toward personalized regenerative medicine—where therapies are tailored to each individual using their own cells.
Imagine a patient with heart disease. Doctors extract a few skin cells, reprogram them into iPSCs, then turn those into healthy heart cells. These are implanted into the patient’s heart, repairing the damage without rejection or side effects. All from their own body.
Or consider a patient with ALS. Scientists create motor neurons from the patient’s iPSCs and use them to test hundreds of drugs in the lab, finding the one that works best for their specific disease profile.
This is the future stem cell researchers are working toward: a world where cells replace pills, and the body becomes its own pharmacy.
Conclusion: Bridging Science and Hope
Stem cells represent one of the most exciting frontiers in modern medicine. They offer the potential to heal, to regenerate, and to fundamentally change how we treat disease. But they are not magic. They are not a universal cure. And they must not be oversold.
The hope is real—but so is the hype. Separating the two requires a commitment to rigorous science, ethical responsibility, and transparent communication.
The story of stem cells is far from over. In many ways, it’s only just begun. But if we continue on this path—with patience, integrity, and imagination—we may one day look back on this era as the dawn of a new age in medicine.
A future not of aging, deterioration, and decline—but of renewal, resilience, and rebirth. A future where the body doesn’t just survive—but knows how to heal itself.
That is the ultimate promise of stem cells—and it’s one worth chasing.