Hormones are powerful messengers in the body. They regulate growth, reproduction, mood, and countless other processes. Yet, their power is a double-edged sword. In recent years, scientists have been investigating how hormonal therapies and contraceptives may affect the risk of certain tumors. One of the latest and most comprehensive studies—conducted by investigators at Case Western Reserve University School of Medicine and Cleveland Clinic—has uncovered a striking connection between depot medroxyprogesterone acetate (dMPA), a commonly used injectable contraceptive, and the risk of meningioma, a type of brain tumor.
The study, published in JAMA Neurology, doesn’t simply add a footnote to medical knowledge—it raises important questions about long-term contraceptive use, women’s health, and how we understand the relationship between hormones and the brain.
Understanding Meningiomas
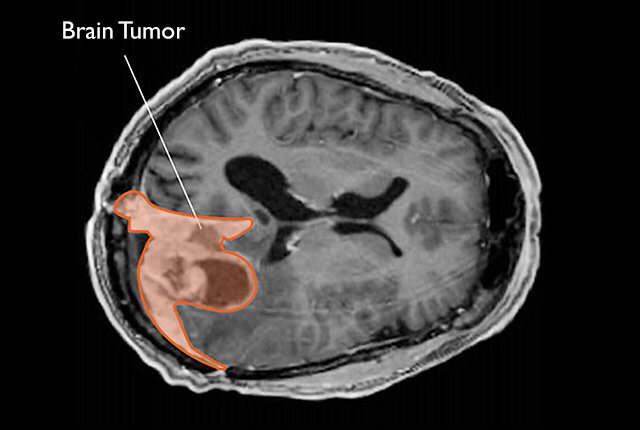
Meningiomas are tumors that form in the meninges, the protective membranes surrounding the brain and spinal cord. They account for nearly 40% of all primary brain tumors in the United States, making them the most common type. While usually classified as non-cancerous, meningiomas can still be dangerous. Their slow but persistent growth can press against critical parts of the brain, leading to headaches, vision problems, seizures, or cognitive changes.
One of the most notable features of meningiomas is their higher prevalence in women. Researchers have long suspected a hormonal link, especially since many meningiomas show progesterone receptor activity and, to a lesser extent, estrogen receptor activity. These observations laid the groundwork for asking a deeper question: could certain hormonal therapies be influencing the risk of developing these tumors?
What Is Depot Medroxyprogesterone Acetate?
Depot medroxyprogesterone acetate, often known by its brand name Depo-Provera, is an injectable form of progestin. It is widely used for contraception and sometimes prescribed for conditions like abnormal uterine bleeding or endometriosis. A single injection provides protection against pregnancy for three months, making it convenient and reliable.
Unlike daily oral contraceptives or localized devices such as intrauterine devices (IUDs), dMPA delivers a high systemic dose of synthetic progesterone. This difference in delivery and hormonal concentration may help explain why its effects on the body are unique—and why researchers turned their attention toward it in relation to meningiomas.
The Study at a Glance
The recent investigation was ambitious in both scope and design. Researchers performed a retrospective, population-based cohort analysis using TriNetX, a massive U.S. healthcare database including data from 68 health systems. The study window spanned from December 2004 to December 2024, providing two decades of medical information.
Out of more than 61 million eligible women, over 10 million were included in the analysis across various exposure groups, ranging from users of dMPA to those relying on oral contraceptives, IUDs, subdermal implants, or progestin-only pills. This scale gave the investigators extraordinary statistical power to detect differences in risk.
The findings were sobering. Women who used depot medroxyprogesterone acetate had a 143% higher risk of being diagnosed with meningioma compared with matched controls. Put differently, the incidence was 7.39 cases per 100,000 patient-years in the dMPA group versus 3.05 in controls.
The Role of Duration and Age
Perhaps the most revealing part of the study was how risk varied depending on the length of use and age at initiation.
Women who used dMPA for four to six years faced a 200% higher risk of meningioma, and those who continued beyond six years experienced a 290% higher risk. This suggests that the cumulative hormonal exposure plays a critical role in tumor development.
Age at initiation also mattered. Starting dMPA between ages 31 and 40 carried a 277% increased risk, while beginning between 41 and 50 meant a 175% increase. For women who started at age 50 or older, the risk rose by 220%. These findings imply that not only duration but also the hormonal environment of the body at different life stages influences susceptibility.
How Do Other Contraceptives Compare?
The study also examined a wide range of other contraceptives for comparison. Oral medroxyprogesterone acetate showed only a modest 18% higher risk, while other methods—including combined oral contraceptives, copper IUDs, progestin-only pills, levonorgestrel IUDs, and subdermal implants—showed no increased risk at all.
In fact, some methods appeared to reduce the likelihood of meningioma. Combined oral contraceptives were associated with a 26% lower risk, and intrauterine devices overall with a 13% lower risk. The 52 mg levonorgestrel IUD showed the most striking protective effect, with a 21% lower risk compared to controls.
These differences reinforce the idea that not all hormones are equal, and not all delivery systems affect the body in the same way. The high-dose, systemic nature of depot injections may be uniquely influential in altering brain tumor risk.
Why Might Progestins Influence Brain Tumors?
The biological explanation lies in the hormone receptors present in meningiomas. Many of these tumors express progesterone receptors, which means they can respond to and be influenced by circulating progesterone or synthetic progestins. In some cases, hormonal exposure may accelerate the growth of cells that have already started to form a tumor.
This does not mean that every woman using dMPA will develop a tumor. Rather, it means that prolonged and late-starting exposure may tip the balance for certain individuals who are already predisposed, either genetically or biologically. Understanding these mechanisms could help scientists not only identify who is most at risk but also guide safer contraceptive practices.
Implications for Women’s Health
The findings of this study carry significant weight because dMPA remains a widely used contraceptive around the world. For many women, especially those who cannot or prefer not to use estrogen-containing methods, dMPA provides a convenient and effective solution. However, the potential trade-off is now clearer: long-term or later-in-life use may increase the risk of developing a brain tumor.
Importantly, the overall risk of meningioma remains relatively rare, even in women using dMPA. A rate of 7.39 cases per 100,000 patient-years means that the vast majority of women will never develop this tumor. Still, for individuals considering long-term contraception, the information empowers them to make more informed choices in consultation with their healthcare providers.
The Bigger Picture of Hormones and Cancer
This study also fits into a broader narrative of how hormones influence cancer and tumor risk. From breast cancer and endometrial cancer to meningiomas, the role of estrogen and progesterone has been scrutinized for decades. Each new study adds to the puzzle, reminding us that the endocrine system is deeply interconnected with cellular growth and repair.
For researchers, the findings highlight the need to distinguish between different types of hormonal therapies, delivery methods, and life stages. For women, the message is not one of alarm but of awareness: hormonal choices have complex ripple effects, and ongoing monitoring is essential.
Looking Ahead
The story of depot medroxyprogesterone acetate and meningiomas is not yet finished. While this study provides the strongest evidence to date of an association, more research is needed to confirm causality, uncover biological mechanisms, and explore strategies to minimize risk. Future studies may also examine whether discontinuing dMPA lowers risk over time, or whether certain subgroups of women are more vulnerable than others.
As science progresses, the hope is not to create fear around contraception but to provide clarity. Every woman deserves both reproductive freedom and long-term health, and research like this ensures that choices are informed by the best possible evidence.
A Balance Between Choice and Caution
The findings from Case Western Reserve University and Cleveland Clinic remind us that medicine is always a balance—between benefits and risks, between convenience and caution. Depot medroxyprogesterone acetate remains an effective and valuable option for many women, but it is no longer possible to ignore its association with meningioma risk.
Ultimately, the power of this study lies in giving women and their doctors the knowledge they need to weigh options. It is a reminder that science is not static but evolving, always deepening our understanding of the connections between the choices we make and the lives we lead.
More information: Tianqi Xiao et al, Depot Medroxyprogesterone Acetate and Risk of Meningioma in the US, JAMA Neurology (2025). DOI: 10.1001/jamaneurol.2025.3011
Gilles Reuter et al, Depot Medroxyprogesterone and Meningioma Risk, JAMA Neurology (2025). DOI: 10.1001/jamaneurol.2025.2973