For decades, biology students have been taught that mitochondria are the “powerhouse of the cell.” These tiny organelles, tucked inside nearly every cell in the human body, are responsible for generating the bulk of cellular energy. But in recent years, scientists have begun to uncover a far more complex role for mitochondria—one that extends beyond powering life and into the dark world of cancer biology.
A new wave of research is revealing that mitochondria are not passive energy machines, but active participants in cancer growth and spread. Now, researchers have identified a surprising culprit: the mitochondrial metabolite glutathione. Their findings suggest that this compound plays a decisive role in helping breast cancer cells break free from the primary tumor, travel through the bloodstream, and colonize distant tissues like the lung.
The discovery represents one of the first direct links between a mitochondrial metabolite and metastasis, the deadliest stage of cancer progression.
The Silent Killer: Metastasis
The true danger of cancer lies not in the primary tumor but in its ability to spread. While surgery, radiation, or targeted therapies may control the original mass, once cancer cells migrate and establish colonies in vital organs, treatment becomes exponentially more difficult. In fact, metastasis is responsible for the vast majority of cancer deaths worldwide.
For decades, scientists have tried to understand the precise cellular machinery that enables metastasis. What gives a single cell the power to detach from its neighbors, survive the harsh journey through the bloodstream, and thrive in the unfamiliar environment of another organ?
Part of the answer lies in metabolism. Cancer cells have long been known to rewire their metabolism to fuel their rapid growth. But now, researchers are finding that these metabolic tricks also help cancer cells spread. Molecules like lactate, pyruvate, glutamine, and serine have each been implicated in supporting metastasis. The mitochondria, which supply not only energy but also a steady stream of metabolites, appear to be at the heart of this transformation.
Following the Trail of Glutathione
To investigate how mitochondria aid metastasis, researchers at Rockefeller University, led by Kivanç Birsoy, developed a new strategy. They tagged cancer cells in such a way that they could distinguish between those that remained in the breast and those that had migrated to the lung. This allowed them to directly compare the metabolic fingerprints of primary tumors and metastatic colonies.
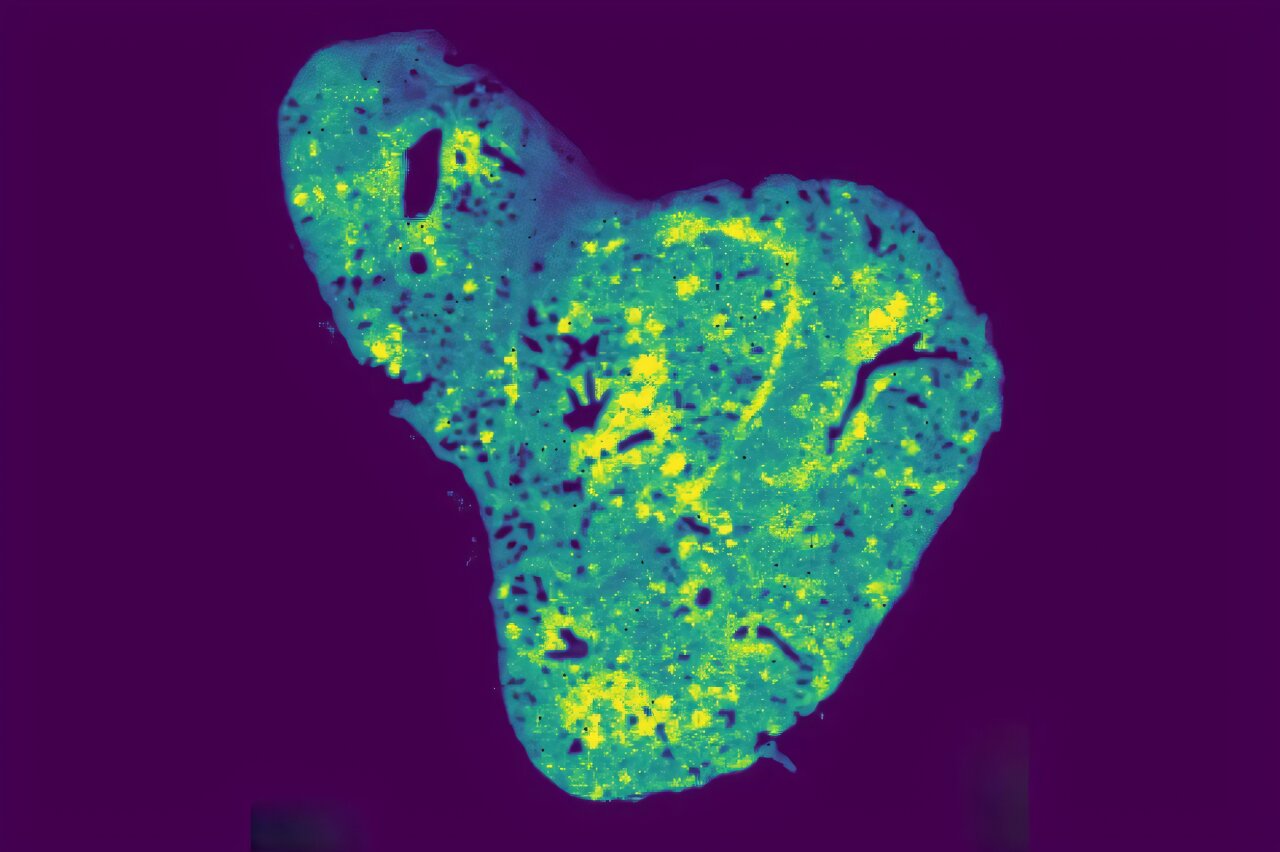
Among thousands of mitochondrial metabolites, one stood out: glutathione. Known as a master antioxidant, glutathione typically protects cells from damage caused by reactive oxygen species. But in the metastatic breast cancer cells that had successfully invaded the lung, glutathione levels were found to be dramatically elevated.
Using cutting-edge spatial metabolomics—a technology that can map molecules directly within tissue samples—the team confirmed that glutathione was concentrated within lung metastases. This suggested that the metabolite was not merely a byproduct but a key player in cancer spread.
A Transporter with a Dark Role
Identifying glutathione was only part of the puzzle. To understand how it was influencing metastasis, the team looked at how the metabolite enters the mitochondria. Their focus quickly turned to SLC25A39, a protein embedded in the mitochondrial membrane.
SLC25A39 had already made headlines in 2021, when Birsoy’s lab discovered that it was the transporter responsible for shuttling glutathione into mitochondria. By 2023, they had also shown that the protein was a dynamic sensor, adjusting glutathione levels as needed. With this background knowledge, the researchers now had the perfect tool for testing glutathione’s role in metastasis.
When they blocked SLC25A39 and prevented glutathione from entering mitochondria, metastatic cancer cells struggled to survive in the lung. This suggested that mitochondrial glutathione was not just present—it was essential.
Surviving Stress in a New Environment
Why does glutathione matter so much during metastasis? The answer lies in stress. When cancer cells arrive in a new tissue, they face an oxygen-poor, nutrient-limited, and hostile environment. Most cells would die under such conditions. But metastatic cells, equipped with elevated glutathione, activate a transcription factor known as ATF4.
ATF4 acts like a survival switch, helping the cells adapt to low-oxygen stress and other environmental challenges. Intriguingly, the researchers found that glutathione’s effect was not due to its usual antioxidant function, but rather its ability to trigger this molecular survival program.
This means that glutathione is not merely protecting cells from damage—it is actively instructing them on how to endure the harsh conditions of metastasis.
Clinical Implications
The implications of these findings extend far beyond the laboratory. When the team analyzed patient samples, they discovered that breast cancer tumors which had spread to the lung showed elevated levels of SLC25A39. Moreover, patients with higher expression of this transporter had poorer overall survival rates.
This raises an exciting possibility: if a drug could be developed to block SLC25A39, it might prevent cancer cells from importing glutathione into mitochondria, thereby cutting off a critical survival mechanism for metastasis. Unlike traditional chemotherapy, which targets rapidly dividing cells broadly, such a therapy might specifically block the deadly spread of cancer with fewer side effects.
While such treatments are still years away, the discovery shifts the way scientists think about cancer metabolism. Rather than focusing solely on bulk cellular metabolism, attention is now turning toward metabolites within specific organelles, like mitochondria.
A New Chapter in Cancer Metabolism
This research underscores a broader shift in the field of cancer biology. For decades, metabolism was seen largely as a background process—a supporting act rather than the main stage. But the growing evidence suggests that metabolic choices made by cancer cells are central to their ability to grow, resist therapy, and metastasize.
By zooming in on mitochondria and their metabolites, scientists are uncovering the fine details of how cancer cells exploit cellular machinery for survival. Glutathione and its transporter SLC25A39 now stand as prime examples of how a single metabolite can tip the balance between life and death for a cancer cell.
Looking Ahead
Much work remains to be done. Researchers still need to determine exactly how glutathione activates ATF4, and whether other metabolites within mitochondria play similar roles in metastasis. Moreover, developing drugs that can safely and specifically block mitochondrial transporters is a formidable challenge.
But the promise is clear. If scientists can learn to disrupt the precise metabolic strategies that cancer cells rely on to metastasize, it could open a new frontier in cancer therapy. Instead of fighting tumors after they spread, medicine might one day prevent them from spreading in the first place.
Conclusion: The Powerhouse Reimagined
The mitochondria may always be remembered as the powerhouse of the cell, but discoveries like these remind us that they are much more. In the context of cancer, mitochondria are not just generating energy—they are shaping destiny. By feeding cancer cells the metabolites they need to colonize new territories, mitochondria become accomplices in metastasis.
The identification of glutathione and its transporter SLC25A39 as central players in this deadly process is a breakthrough with both scientific and clinical significance. It opens a path to rethinking cancer treatment at the level of organelles and their metabolites, shifting our focus from broad strokes to fine details.
In the story of cancer, where survival often hinges on a single cell’s ability to adapt, mitochondria emerge not just as powerhouses, but as hidden architects of fate.
More information: Hsi-wen Yeh et al, Mitochondrial glutathione import enables breast cancer metastasis via integrated stress response signaling, Cancer Discovery (2025). DOI: 10.1158/2159-8290.CD-24-1556