Depression is often described as a heavy shadow that falls across a person’s life. It slows the body, disturbs sleep, clouds thoughts, and gradually isolates individuals from the world around them. It is not merely a passing sadness, but a serious disorder that can rob life of meaning and, at its most severe, increase the risk of suicide. According to global health data, more than 280 million people worldwide now live with depression as of 2025—a staggering number that continues to rise year after year.
Despite decades of research, depression remains one of the most complex mental illnesses to understand and treat. Traditional therapies focus largely on neurotransmitters, especially serotonin, and yet antidepressants help only about half of patients. Many are left struggling with lingering symptoms or side effects that create new challenges. The urgency for new approaches has never been greater, and recent research may mark a turning point in how we think about the biology of depression.
A New Clue from the Brain
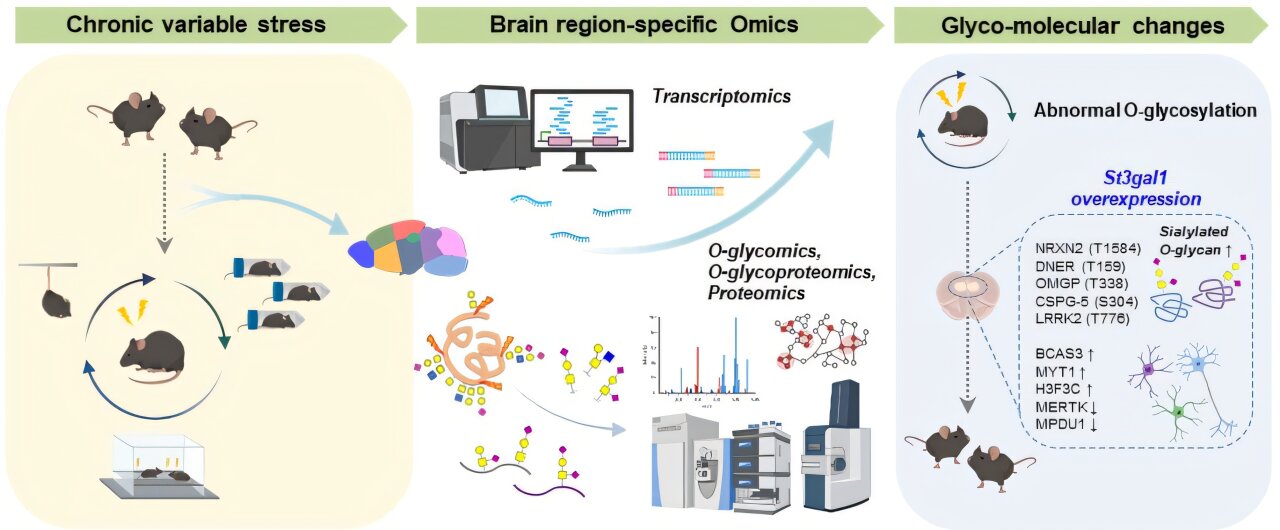
In a groundbreaking study published in Science Advances, researchers from the Institute for Basic Science (IBS), led by C. Justin Lee and Lee Boyoung, have uncovered a previously hidden molecular pathway linked directly to depression. Their discovery focuses not on neurotransmitters but on sugar modifications in the brain—a field known as glycosylation.
At first, the connection between sugar chains and mood disorders may sound unexpected. Yet glycosylation, the process by which sugars attach to proteins and influence their structure and stability, plays a fundamental role in nearly every biological system. It has long been implicated in cancer, viral infections, and neurodegenerative conditions. Only recently have scientists begun to ask what role glycosylation might play in mental health, and the answers are proving both surprising and profound.
Sugar Chains and the Brain’s Balance
The IBS team examined glycosylation across different brain regions using high-performance mass spectrometry, a powerful tool that can detect subtle molecular changes. They found that each brain region has its own distinctive glycosylation “signature,” much like a fingerprint. This baseline is important because it establishes what healthy brain patterns look like.
When the team compared healthy mice with mice subjected to chronic stress—a common model for studying depression—they discovered striking differences. In particular, the prefrontal cortex, a region critical for emotion regulation and decision-making, showed disruptions in O-glycosylation. One modification stood out: a reduction in sialylation, the addition of sialic acid molecules that normally stabilize proteins.
This change was tied to a drop in the enzyme St3gal1, which is responsible for attaching sialic acid to sugar chains. Without enough St3gal1, protein structures became unstable, and neural circuits began to falter.
Turning Genes On and Off
To test the direct link between St3gal1 and depression, the researchers went further, manipulating the enzyme in living mice. The results were dramatic. When St3gal1 was suppressed in normal mice, they began to show depressive-like behaviors, such as a loss of motivation and heightened anxiety, even without stress. Conversely, when St3gal1 expression was boosted in stressed mice, their depressive symptoms eased.
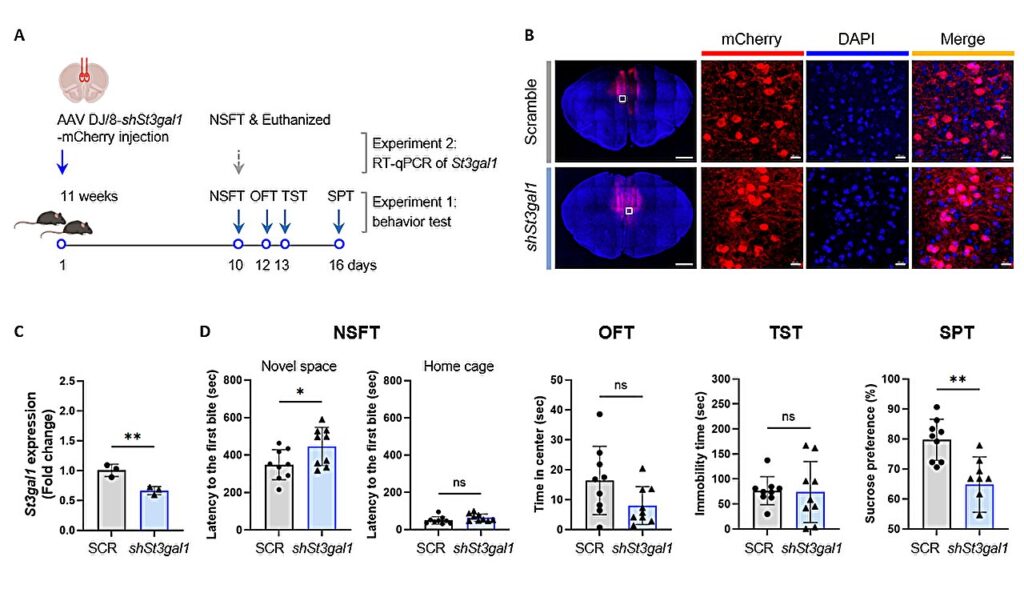
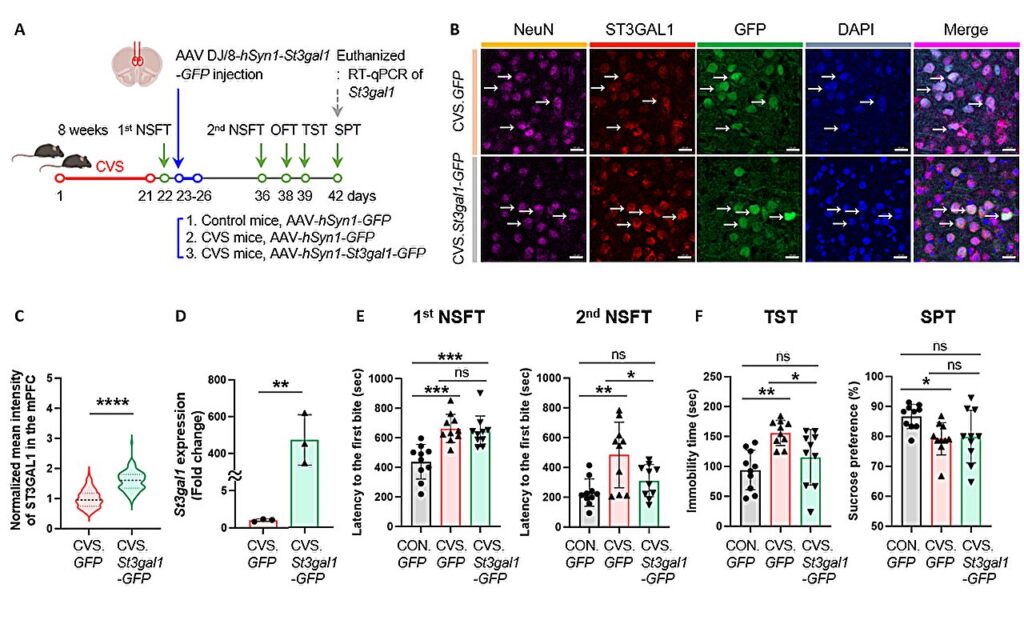
This clear cause-and-effect relationship was groundbreaking: it demonstrated that changes in sugar chain processing alone, independent of external stress, could trigger depression. St3gal1 emerged as a key molecular switch in the brain’s emotional balance.
Synapses Under Stress
The team’s deeper analyses revealed how this mechanism disrupts brain circuits. Reduced St3gal1 altered the glycosylation of synaptic proteins, especially neurexin 2 (NRXN2), a molecule that helps neurons connect and communicate. Without stable sugar chains, inhibitory neurons—those responsible for calming and balancing brain activity—lost their effectiveness.
Imagine a symphony where violins and trumpets play louder and louder while the conductor loses control over the orchestra. The balance collapses, and the music turns into chaos. In much the same way, when inhibitory neurons fail, the brain’s emotional circuits spiral into dysregulation, giving rise to depressive states.
Beyond Neurotransmitters
The implications of this study are profound. For decades, the dominant theory of depression has centered on chemical imbalances of neurotransmitters like serotonin, norepinephrine, and dopamine. While this model has guided the development of antidepressants, its limitations are painfully clear. Half of patients do not respond to treatment, and many experience side effects such as gastrointestinal distress or increased anxiety.
The discovery of glycosylation as a molecular pathway opens up a new frontier. By targeting sugar modifications and enzymes like St3gal1, future therapies may be able to address depression from an entirely different angle, offering hope to those who have not benefited from existing treatments.
A Wider Horizon of Mental Health
Depression is rarely an isolated illness. It often overlaps with anxiety disorders, post-traumatic stress disorder (PTSD), and schizophrenia, creating a web of suffering that affects not only individuals but also families and societies. Director C. Justin Lee emphasized that this new understanding of glycosylation may extend beyond depression itself, paving the way for therapies across a spectrum of psychiatric conditions.
The potential is enormous. If we can identify biomarkers based on sugar modifications, early diagnosis of depression could become more precise. Treatments could be tailored not just to symptoms but to underlying molecular patterns, creating a more personalized approach to mental health care.
The Human Dimension
Behind every scientific discovery lies a human story. Depression is not simply a collection of symptoms—it is an illness that drains lives of joy and purpose. Families struggle to understand loved ones who withdraw from them, workers lose careers to exhaustion and lack of motivation, and young people face futures clouded by hopelessness.
The rise in depression cases worldwide reflects both the pressures of modern life and the deep biological vulnerabilities we carry. Research like the IBS study gives reason for cautious optimism. It reminds us that the brain, complex as it is, operates on principles that science can uncover. Every new pathway revealed is not just a scientific achievement but a step toward relief for millions of people.
Toward a Future of Healing
The discovery of glycosylation’s role in depression is still young, and much work remains before it can be translated into clinical therapies. Yet it represents a paradigm shift, a widening of the lens through which we view mental illness. No longer is depression explained solely by neurotransmitters; now we see the intricate dance of proteins and sugar chains shaping the brain’s circuits and emotions.
This insight is not an end but a beginning. With further research, the molecular story of depression will grow clearer, guiding us toward treatments that are more effective, more precise, and kinder to patients. The hope is not only to alleviate symptoms but to restore balance, resilience, and vitality to lives interrupted by depression.
Conclusion: Light Through the Darkness
Depression has always been difficult to describe because it is both a medical disorder and a deeply personal experience. For some, it feels like numbness; for others, an unbearable weight. Its reach is vast, touching every continent, every community, every age group. But science continues to push back against the darkness.
The discovery of abnormal glycosylation in the brain’s prefrontal cortex offers a new thread of understanding. It tells us that even the smallest molecules—the sugars decorating our proteins—play a role in the vast emotional landscapes of the human mind. And in that knowledge lies hope: that through curiosity, persistence, and compassion, we can illuminate the hidden mechanisms of suffering and, one day, lift the burden of depression from millions of lives.
More information: Youngsuk Seo et al, Abnormal O-Glycan Sialylation in the mPFC Contributes to Depressive-like Behaviors in Male Mice, Science Advances (2025). DOI: 10.1126/sciadv.ady2733.