In a groundbreaking study that challenges long-held beliefs about how our bodies manage fat, scientists from the University of Birmingham have uncovered a completely new mechanism by which fat cells regulate the release of energy. Published today in Nature Chemical Biology, the discovery unveils a powerful self-regulating system that takes place not at the cell surface—as previously thought—but deep inside the fat cell itself.
This revelation doesn’t just rewrite textbook biology. It opens a new frontier in the fight against obesity, type 2 diabetes, and related metabolic diseases, offering a radically more precise way to manipulate fat metabolism.
From Static Storage to Dynamic Decision-Making
Fat cells—formally known as adipocytes—have long been seen as passive storage units: repositories of energy that quietly hold onto fat until the body signals a need for fuel. When energy is required, the process of lipolysis kicks in, breaking fat down into usable fatty acids. But too much lipolysis, and you end up with dangerous levels of fat in the blood; too little, and your body can’t access the energy it needs.
Scientists have known that the process is controlled by receptors, the most important of which are G protein-coupled receptors (GPCRs) located on the surface of the cell. These receptors listen for external cues—like dietary fats—to tell the fat cell when to store and when to release.
But what if the real decision-makers aren’t on the cell surface at all?
A Receptor That Hides in Plain Sight
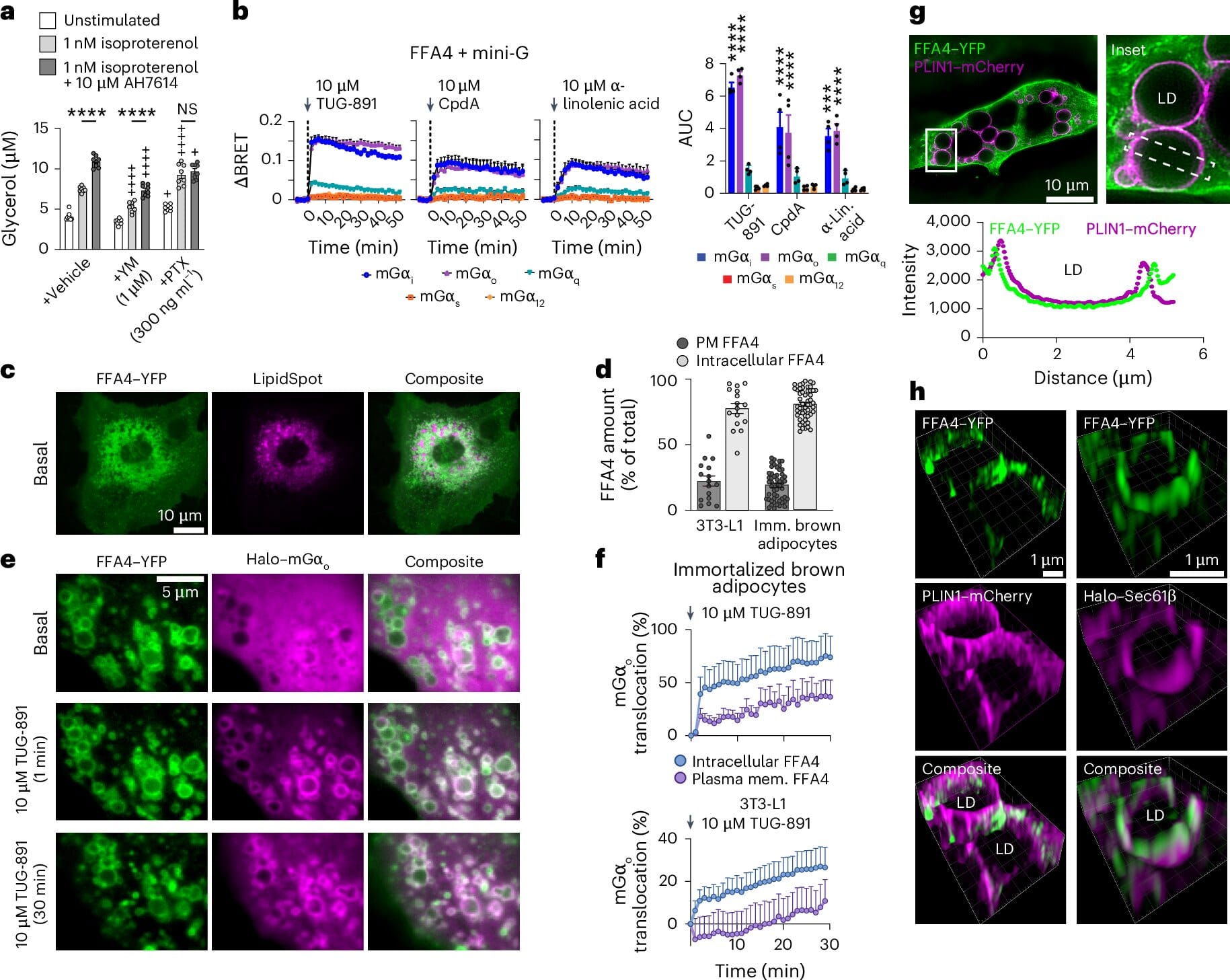
The new study, carried out in collaboration with researchers at the Universities of Copenhagen, Glasgow, and Montréal, focused on a specific receptor called free fatty acid receptor 4 (FFA4), a GPCR long known to respond to dietary fats.
What the researchers found, however, upends the traditional view. Using cutting-edge imaging and molecular techniques, they discovered that FFA4 is not primarily located on the outer membrane of the fat cell, as expected, but rather inside it—strategically positioned on internal membranes that wrap around tiny fat-storage units known as lipid droplets.
These droplets are the heart of the fat cell’s storage system, like fuel tanks waiting to be tapped. When fat is broken down into fatty acids, these newly released molecules activate the FFA4 receptors sitting right next to the storage sites. In response, FFA4 signals the cell to slow down fat breakdown—a built-in feedback mechanism that acts like an emergency brake, preventing runaway fat release.
A Discovery That Redefines Cell Signaling
This mechanism—called intracrine signaling—is a revelation. It means the fat cell doesn’t wait for outside instructions to regulate itself. Instead, it listens to its own internal changes and acts instantly, with precise, localized control.
“We were surprised to find that FFA4 works like a built-in sensor inside fat cells,” said Dr. Shannon O’Brien, research fellow at the University of Birmingham and co-lead author of the study. “It’s a real-time monitoring system that helps cells avoid dangerous extremes in fat metabolism. This kind of localized, fast-acting feedback loop has never been described before in metabolite-sensing receptors.”
While intracrine signaling has been suggested in hormonal systems before, this is the first time it has been directly observed in the realm of fat metabolism—and in such a strikingly specific way.
Implications for Obesity, Diabetes, and Beyond
The implications are enormous. If fat cells have their own internal brake system, then targeting that system directly could offer far more refined and effective therapies for metabolic diseases. Rather than flooding the body with drugs that affect every cell indiscriminately, treatments could be designed to target only the internal FFA4 receptors in adipocytes.
“This discovery could change the way we think about drug development for metabolic diseases,” said Professor Davide Calebiro, senior author of the study and expert in molecular endocrinology at the University of Birmingham. “By focusing on the intracellular version of FFA4, we may be able to develop more precise medications that better control fat metabolism without the side effects caused by affecting other tissues.”
Such medications could help people with type 2 diabetes by better regulating blood lipid levels, or assist individuals with obesity by fine-tuning how their fat cells release energy. The potential benefits extend beyond fat tissue, too, since GPCRs like FFA4 are found throughout the body and may engage in similar intracrine behavior elsewhere.
The Cell’s Private Conversation, Now Public
Until now, the scientific focus has been on how cells respond to external messages—from hormones, nutrients, or neural signals. But this discovery shifts the spotlight inward. The fat cell isn’t just a listener. It talks to itself.
The research reveals that adipocytes can rapidly and locally adjust their behavior in response to their own activity. This makes the cell far more sophisticated than previously thought—not just a warehouse, but a command center of energy regulation.
“This completely novel mechanism of ‘intracrine’ signaling is likely just the tip of the iceberg,” Professor Calebiro added. “It may apply to many other metabolically important receptors, not just FFA4. We’re looking at an entirely new layer of cell communication that has been hidden in plain sight.”
A New Era of Metabolic Medicine
The discovery has already sparked interest among drug developers, who are eager to explore the possibility of designing intracellular-targeting compounds—medications that could fine-tune lipid metabolism with surgical precision.
And it’s not just about treating disease. This deeper understanding of how fat cells operate could also inform lifestyle interventions, such as diet and exercise, by showing how certain nutrients or physical activities may influence these internal receptor systems.
In the long term, the team believes this discovery could catalyze a new generation of metabolic research—one focused not just on what enters the cell, but on what happens next, deep within its inner architecture.
The Hidden Intelligence of Fat
For decades, fat tissue has been a biological underdog—often vilified, rarely admired. But science is revealing fat as a dynamic, intelligent, and deeply integrated organ system. It senses, it responds, and now we know: it self-regulates.
“This discovery reminds us that even the most familiar parts of our biology still hold mysteries,” Dr. O’Brien said. “Sometimes, the biggest breakthroughs come not from looking farther out, but from looking deeper in.”
With obesity and metabolic disorders affecting hundreds of millions worldwide, the timing of this discovery couldn’t be more urgent. Understanding how fat cells talk to themselves may be the key to finally learning how to help them listen—to us.
More information: Shannon L. O’Brien et al, Intracrine FFA4 signaling controls lipolysis at lipid droplets, Nature Chemical Biology (2025). DOI: 10.1038/s41589-025-01982-5