Beneath the calm rhythm of digestion, beneath every swallowed bite and every chemical breakdown, a hidden world hums with extraordinary precision. It is not just the domain of enzymes and nutrients, but of tireless cellular choreography—a place where the tiniest actors make life-sustaining decisions every moment.
At the heart of this secret world lies the intestinal lining—arguably one of the most regenerative tissues in the human body. Every few days, its cells are reborn. Millions slough off, and millions more rise to take their place. This near-constant renewal is made possible by a small, elite group of stem cells nestled in microscopic pockets within the gut lining called crypts.
But stem cells are not autonomous. They wait patiently in their specialized niche, listening. What they become—whether a mucus-producing goblet cell, a nutrient-absorbing enterocyte, or another vital gut cell—depends entirely on the instructions they receive from their environment. For decades, scientists believed these instructions drifted randomly through tissue, diffusing like perfume in a room, eventually reaching their target cells. It was a model of organized chaos. It worked—but only just.
Now, in a startling twist that rewrites the very rules of gut biology, researchers in Singapore have discovered that the gut is far less chaotic and far more intentional. Stem cells are not merely eavesdropping on a chemical fog—they are being whispered to with astonishing precision.
A Conversation Hidden in Plain Sight
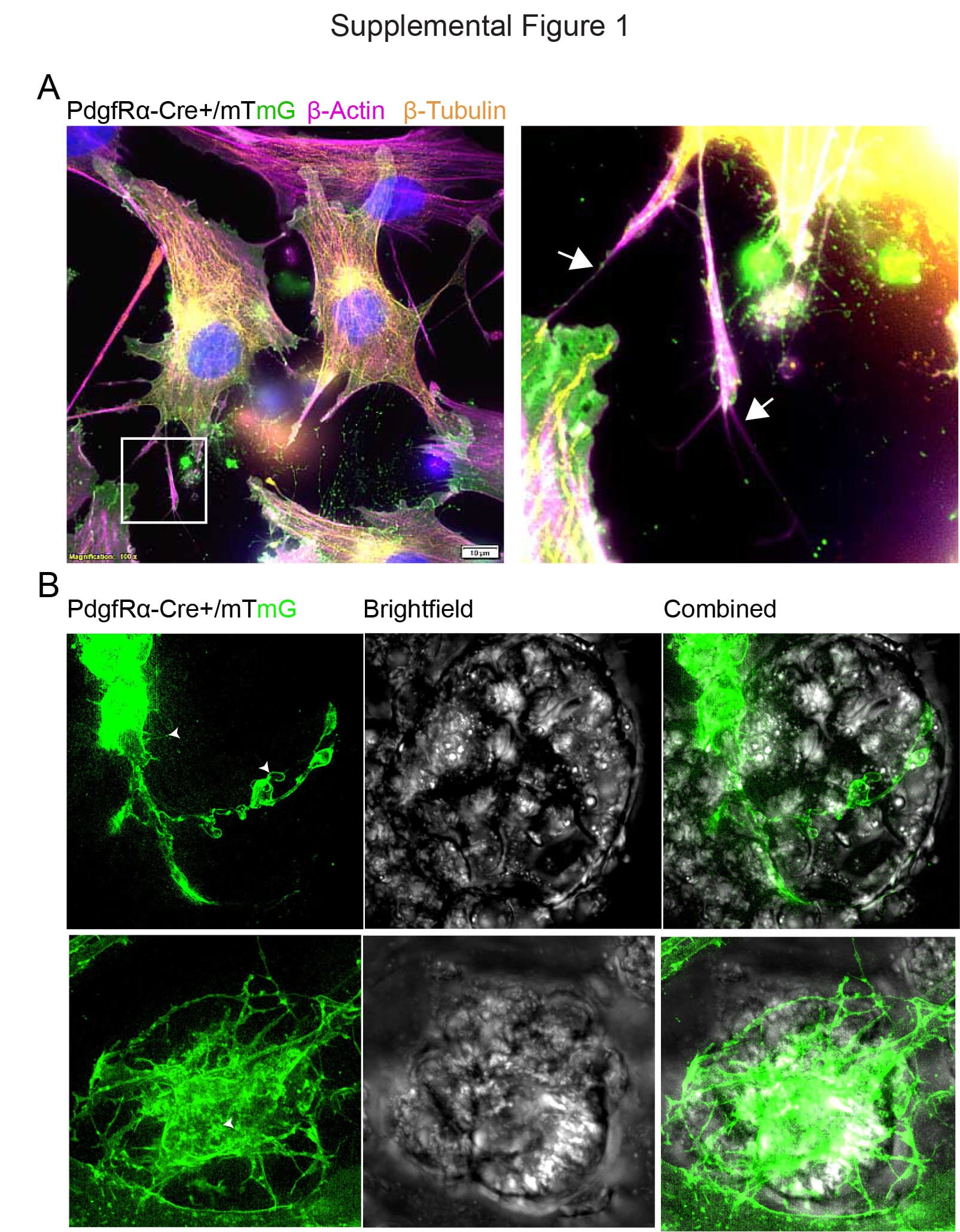
In a groundbreaking study published in Developmental Cell, a team from Duke-NUS Medical School and Nanyang Technological University (NTU Singapore) has revealed a direct, neuron-like system of communication in the gut. Their discovery centers around enigmatic support cells known as telocytes, which use delicate, finger-like extensions called cytonemes to reach out across the crypts and deliver messages to stem cells with pinpoint accuracy.
These messages aren’t trivial. They are made of Wnts—a family of powerful signaling proteins that tell stem cells when to divide, when to specialize, and how to maintain the balance that keeps the gut lining both healthy and adaptable. Until recently, scientists assumed that Wnts simply leaked out of nearby cells and reached stem cells by diffusion. But this new discovery paints a far more sophisticated picture.
“We discovered that these signals aren’t just drifting through tissue,” said Professor David Virshup, a leading stem cell biologist at Duke-NUS and co-author of the study. “They’re being delivered with surprising precision from telocytes directly to stem cells—changing the way we think about how the gut communicates with itself.”
This isn’t just biology—it’s symphony. It’s a neural network made of gut cells. And it is unlike anything scientists had seen before.
Neuron-like Signals in the Belly of the Body
To witness this secret communication, the researchers used a suite of cutting-edge tools—high-resolution fluorescence imaging, electron microscopy, and novel protein tagging systems. What they saw was breathtaking: telocytes extending long, slender arms through the intricate latticework of intestinal tissue, reaching out like neurons in the brain.
These extensions, cytonemes, created one-to-one contact points with stem cells—connections that bore a striking resemblance to synapses, the specialized junctions between neurons where neurotransmitters leap across gaps to send messages.
“It’s a striking example of how imaging at different scales, combined with new molecular tools, can uncover hidden systems we didn’t even know to look for,” said Assistant Professor Alexander Ludwig from NTU Singapore, one of the study’s key authors.
But this was no illusion. At the points where telocytes touched stem cells, researchers observed bundles of Wnt molecules, ready for dispatch. This wasn’t random diffusion. It was direct, deliberate, and astonishingly personal.
The Machinery Behind the Messenger
Of course, precision requires infrastructure. How does a cell grow a communication wire across tissue, aim it perfectly, and deliver life-altering signals?
To understand the construction of these biological message cables, the team focused on the molecular scaffolding that supports cytonemes. Two proteins—KANK and Liprin—emerged as essential players. Without them, the cytonemes couldn’t form. And without cytonemes, the Wnts never reached their intended targets.
“It was like discovering the switches in a telephone exchange,” said Dr. Gediminas Greicius, lead author and principal research scientist at Duke-NUS. “Disabling just a few key proteins stopped the whole communication line from functioning.”
That level of vulnerability is both profound and promising. If we can understand these lines, we might one day repair them. Or even reroute them.
From Healthy Gut to Diseased Tissue
Although the study focused on healthy intestinal tissue, the implications stretch far into the world of disease. The gut, after all, is not merely a digestive organ. It is an immune barrier, a neurological interface, and a hormonal factory. When its communication systems fail, the consequences can be severe.
Disrupted Wnt signaling is already a known contributor to colon cancer, where uncontrolled cell growth may result from stem cells receiving too many “go” signals or not enough restraint. Similarly, in conditions like inflammatory bowel disease (IBD)—which includes Crohn’s disease and ulcerative colitis—improper tissue regeneration and immune misfiring are common themes. Could faulty telocyte-stem cell communication be part of the story?
“If we can understand where and how these precise interactions break down,” said Professor Patrick Tan, senior vice-dean for research at Duke-NUS, “we may be able to restore them—or even improve upon them in the context of regenerative medicine.”
That’s a bold but logical leap. If telocytes can be coaxed to regrow their cytonemes, or if synthetic Wnt carriers could be guided to stem cells with similar precision, a new era of targeted gut repair might be within reach.
A New Blueprint for Stem Cell Therapies
What makes this discovery so electrifying is not just the elegance of the system, but its translational potential. Stem cell therapies are already being explored for a variety of gut-related conditions—from colitis to graft-versus-host disease. But one of the major challenges has always been control. How do you make sure transplanted stem cells behave the way they’re supposed to? How do you make sure they receive the right instructions?
This discovery gives researchers a new blueprint—one rooted in specificity, timing, and physical contact. It suggests that the future of regenerative medicine won’t just be about putting the right cells in the right place, but about making sure they are plugged into the right signals at exactly the right time.
And that, as the Singaporean researchers have now shown, may depend on the unassuming but mighty telocyte—a cell that whispers life into the gut.
The Power of Looking Closer
In the end, this breakthrough is a testament to the beauty of basic science. It did not begin with a grand promise to cure disease. It began with a question: How do cells really talk to each other? The answer—hidden for decades in plain sight—required patience, precision, and faith in the unseen.
“Sometimes, when you study the basics closely, you uncover something transformative,” said Assistant Professor Greicius. “This system of targeted signaling was hiding in plain sight, and now that we see it, it reshapes our understanding of stem cells in the gut.”
That reshaping is already underway. Across disciplines—from cancer biology to tissue engineering—scientists are now rethinking old assumptions. If the gut uses direct communication akin to the brain, how many other organs might do the same? What other overlooked cells might hold the keys to regeneration, repair, and health?
Listening to the Gut, Literally
For centuries, we’ve spoken of “gut feelings” as metaphors. But as science continues to decode the intricate biology of the intestine, we may discover that the gut has been speaking all along—just not in words.
Through the tiny arms of telocytes, through the touch of one cell on another, through the quiet dispatch of Wnt signals—our gut is not only digesting our meals but maintaining a delicate balance of life through an invisible whispering network.
Now that we’ve learned to hear it, the question becomes: what will we do with that knowledge?
Reference: Gediminas Greicius et al, Telocytes deliver essential Wnts directly to murine intestinal stem cells via synapse-like contacts, Developmental Cell (2025). DOI: 10.1016/j.devcel.2025.06.040