Among the many faces of breast cancer, one form stands out as particularly fierce: triple negative breast cancer, or TNBC. Unlike other breast cancers, TNBC does not carry receptors for estrogen, progesterone, or the HER2 protein—the very markers that make many targeted therapies possible. Without these receptors, the most effective precision treatments are stripped away, leaving patients with limited options. Standard chemotherapy and radiation often remain the only lines of defense, and even then, TNBC has a reputation for returning with devastating force.
For many patients, the fear is not just the presence of the initial tumor but the looming possibility of metastasis—the spread of cancer to distant organs such as the lungs, liver, or brain. Once TNBC begins to travel, survival odds decline sharply. It is this aggressive tendency to spread that makes TNBC such a formidable enemy in oncology.
Yet in the midst of this difficult reality, a team of researchers at Weill Cornell Medicine has uncovered something remarkable: a new molecular pathway that could stop TNBC cells from spreading, and perhaps offer patients a desperately needed weapon of hope.
Cracking the Code of Metastasis
The study, published October 2 in Cancer Discovery, focused on a protein called EZH2. Normally, EZH2 functions as part of the cell’s epigenetic machinery—the system that regulates how DNA is packaged and which genes are turned on or off. But in cancer, many normal cellular processes are hijacked.
Dr. Vivek Mittal, the senior author of the study, explained the stakes with striking clarity: “Metastasis is the main reason patients with triple negative breast cancer face poor survival odds. Our study suggests a new therapeutic approach to block metastasis before it starts and help patients overcome this deadly cancer.”
What his team found challenges some long-held assumptions in cancer biology. For years, one strategy against tumors has been to push cancer cells into making so many mistakes during cell division that they collapse under their own chaos. But this approach is risky. Cancer cells thrive on instability; too much chaos can sometimes paradoxically make them more aggressive.
Instead of fueling more instability, the Weill Cornell team discovered that restoring order—by blocking EZH2—may be the key to stopping metastasis at its source.
The Dance of Dividing Cells
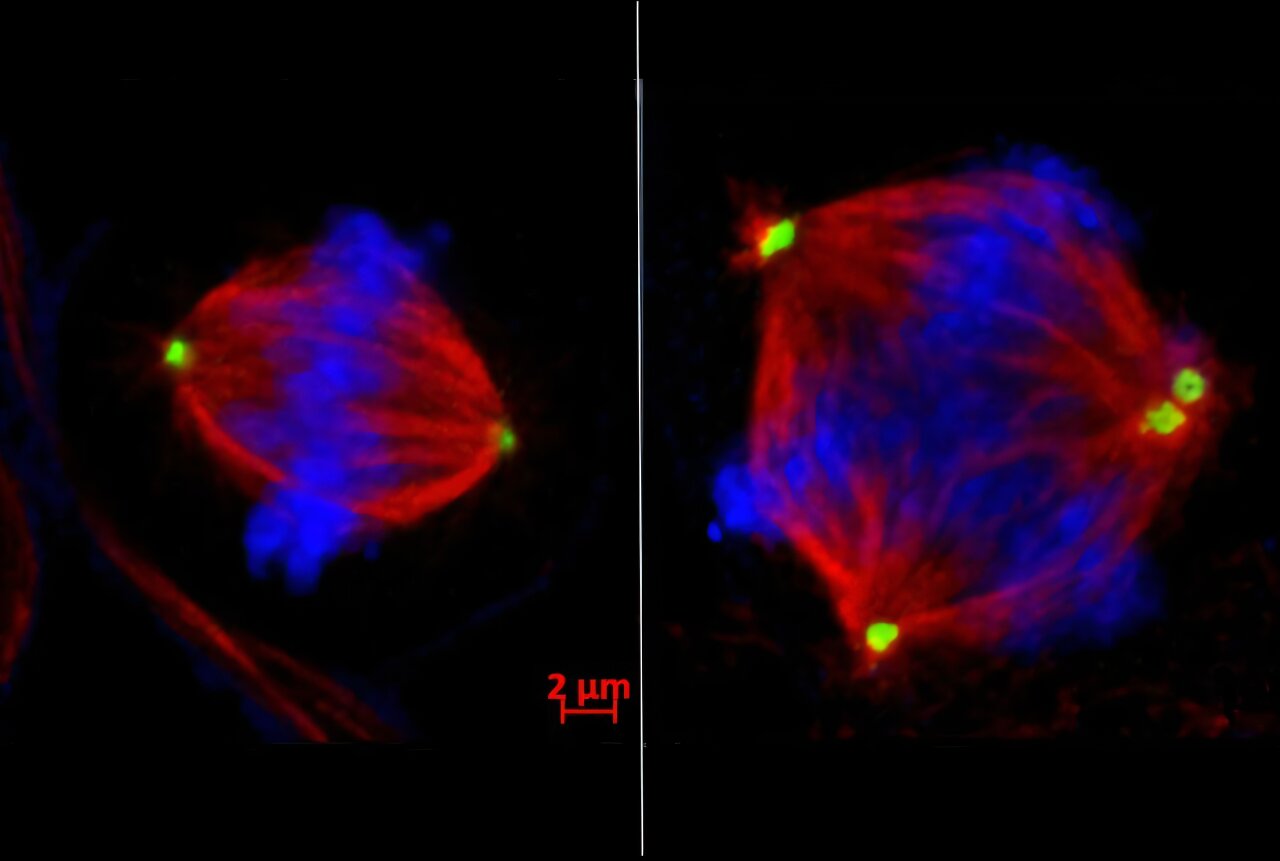
To understand why this matters, it helps to picture the extraordinary process of cell division. Each cell must duplicate its DNA, then carefully separate chromosomes into two equal sets. This is not a casual process; it is one of life’s most intricate dances, choreographed by structures called centrosomes, which pull chromosomes apart like tiny cellular ropes.
But in TNBC cells, this dance often goes awry. Chromosomes end up jumbled, duplicated, or lost, producing cells with genetic chaos. This condition, called chromosomal instability, is a hallmark of aggressive cancers.
Dr. Shelley Yang Bai, the first author of the study, found that EZH2 is directly responsible for this instability in TNBC. By silencing a critical gene called tankyrase 1, EZH2 sets off a domino effect. Without tankyrase 1, another protein called CPAP accumulates in excess. The result: centrosomes multiply wildly, pulling chromosomes into three or more daughter cells instead of two. The order of life’s dance collapses into chaos—and chaos, in the case of cancer, fuels its ability to spread.
The Breakthrough Discovery
In preclinical experiments, the team found that drugs blocking EZH2 could restore balance to this process. By inhibiting EZH2, they prevented centrosomes from multiplying uncontrollably, reduced chromosomal instability, and most importantly, stopped cancer cells from metastasizing.
This is groundbreaking because it links an epigenetic regulator—EZH2—with chromosomal instability in a direct, mechanistic way. For the first time, researchers have shown that you can suppress metastasis by bringing stability back to dividing cells, rather than pushing them further into disorder.
The implications go beyond laboratory findings. Tazemetostat, an FDA-approved drug for other cancers, was shown to have this stabilizing effect. If it can be repurposed for TNBC, patients might gain access to a new treatment much faster than if an entirely new drug needed to be developed from scratch.
From the Lab to the Patient
While the findings are still in the preclinical stage, their potential impact is enormous. TNBC affects thousands of patients each year, disproportionately striking younger women and women of African or Hispanic descent. The aggressiveness of TNBC and the lack of targeted therapies have left many patients with few choices and poor odds.
Now, with EZH2 inhibitors on the horizon, there is hope that doctors may be able to intercept metastasis before it takes hold. As Dr. Magdalena Plasilova, a surgical oncologist and co-author of the study, put it: “I see firsthand the devastating impact of metastases on patients, and this offers hope for improved outcomes and survival rates.”
Linking Epigenetics to the Spread of Cancer
This study also highlights the power of epigenetics in cancer. Unlike mutations, which change the DNA sequence itself, epigenetic changes control how genes are expressed without altering the underlying code. In TNBC, EZH2 essentially rewires the cell’s epigenetic programming to favor instability and metastasis.
By targeting this programming, researchers are not simply attacking cancer cells blindly. They are aiming at one of the master switches that controls whether cancer remains localized or spreads to new organs. This precision offers the possibility of therapies that are more effective and less toxic than conventional treatments.
Looking Beyond TNBC
The implications of this discovery may extend well beyond triple negative breast cancer. Other cancers, such as lung adenocarcinoma, are also marked by chromosomal instability. If EZH2 inhibitors prove effective across multiple tumor types, they could become a universal tool in the fight against some of the deadliest forms of cancer.
Dr. Mittal and his colleagues are already planning collaborations to test the safety of EZH2 inhibitors in clinical trials for breast cancer patients. The road from laboratory bench to bedside is never simple, but the path is clearer now than it was before.
A Shift in Strategy
Perhaps the most profound lesson from this study is not only the promise of a new drug target but a shift in how we think about fighting cancer. Instead of pushing cancer cells toward the brink of collapse by amplifying their errors, scientists may now consider the opposite: restoring order, stabilizing the machinery of cell division, and cutting off cancer’s ability to metastasize at its root.
It is a subtle but powerful reframing—one that reflects a deeper understanding of the biology of cancer. And for patients, it represents a shift from despair to hope, from inevitability to possibility.
The Road Ahead
Triple negative breast cancer remains one of medicine’s most daunting challenges. But discoveries like this one remind us that cancer is not invincible. By peeling back the layers of cellular chaos and uncovering the hidden switches that drive metastasis, researchers are lighting new paths toward survival.
As the science moves forward, the message for patients is clear: there is reason to hope. The battle against TNBC is far from over, but with each breakthrough, the balance tips further toward life.
In the end, this story is not just about enzymes and chromosomes, but about human resilience—the determination of scientists to pursue answers, the courage of patients to face uncertainty, and the possibility that even the most aggressive cancers can one day be brought to heel.
More information: Yang Bai et al, Epigenetic regulation of chromosomal instability by EZH2 methyltransferase., Cancer Discovery (2025). DOI: 10.1158/2159-8290.cd-25-0947