Alzheimer’s disease (AD) is often described as the “silent thief” of memory. It begins quietly, with small lapses—misplaced keys, forgotten names, a momentary struggle to recall a familiar route. Over time, these slips deepen into something far more devastating: the gradual unraveling of identity, language, independence, and connection.
Around the world, millions of families face the slow heartbreak of watching loved ones fade before their eyes. Each year, between 500,000 and 900,000 people are diagnosed with Alzheimer’s, joining the growing population affected by dementia and age-related cognitive decline. Despite decades of research, there is still no cure—only treatments that may slow progression in its early stages.
Yet hope persists. Scientists are not standing still. Every day, researchers peer into the deepest mysteries of the brain, searching for hidden mechanisms that might explain why Alzheimer’s develops and how we might one day stop it. And one unlikely hero keeps emerging from this story: a tiny but mighty brain cell known as the microglia.
Microglia: The Brain’s Watchful Guardians
To understand why microglia are so important, imagine the brain as a bustling city. Neurons are the citizens, transmitting signals like conversations across synapses. Blood vessels are the highways, carrying oxygen and nutrients. But cities also need caretakers—those who clear debris, fight off intruders, and keep things running smoothly. That’s where microglia come in.
Microglia are the brain’s resident immune cells. They patrol tirelessly, scanning for damaged neurons, infectious agents, or harmful debris. When trouble arises, they spring into action—swallowing unwanted particles, releasing protective molecules, and coordinating with other cells to restore balance.
In a healthy brain, this system works beautifully. But in Alzheimer’s, microglia appear to go off course. Instead of helping, they may become overactive, releasing harmful chemicals, or too sluggish, failing to clear toxic protein clumps like beta-amyloid and tau. Either way, their dysregulation contributes to neurodegeneration, accelerating the breakdown of memory and cognition.
Understanding exactly how microglia shift from guardians to disruptors has become one of the most urgent questions in Alzheimer’s research.
A Landmark Study: Looking Deep Into Microglia
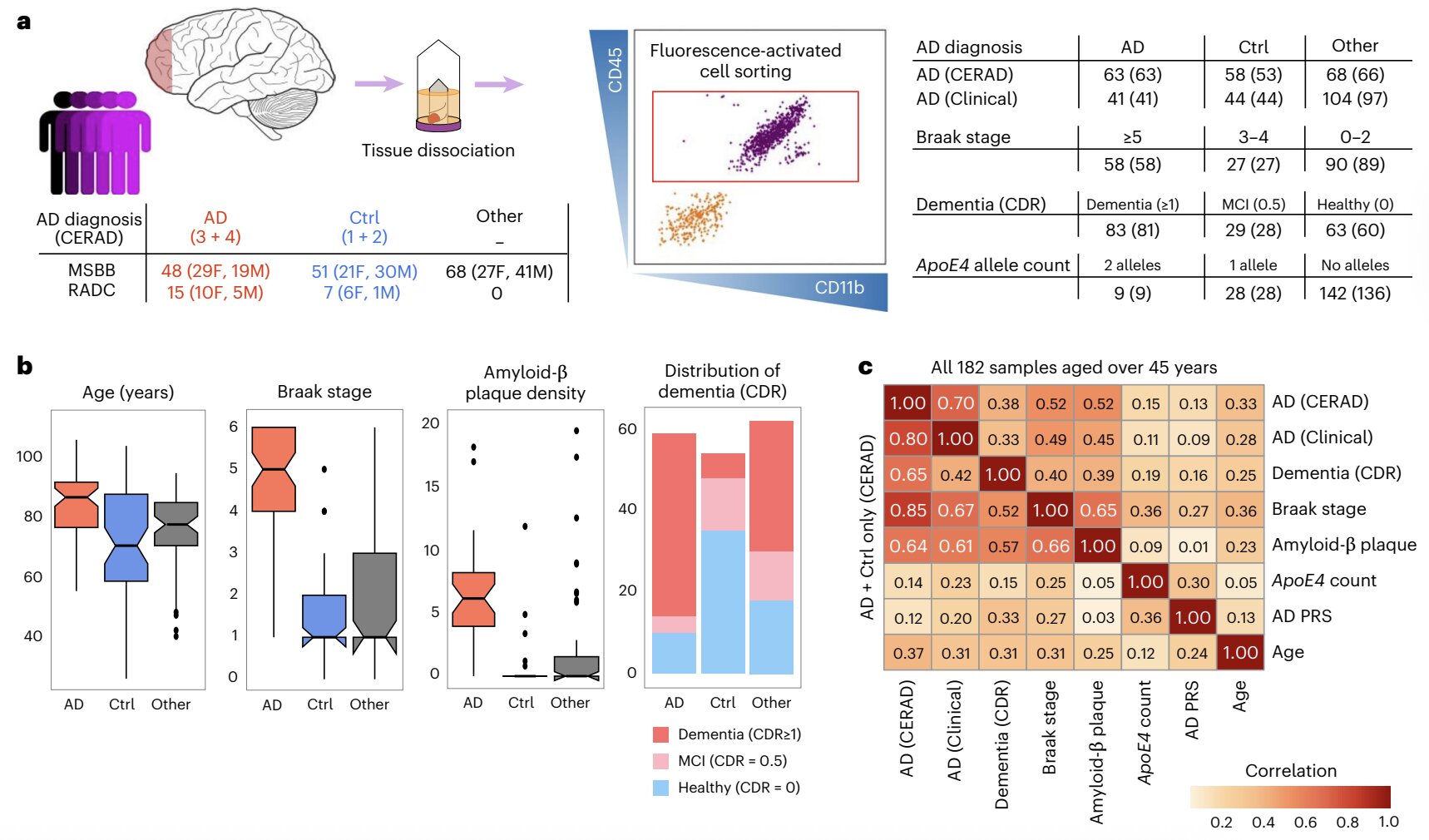
Recently, scientists at the Icahn School of Medicine at Mount Sinai took a bold step toward answering this question. Their work, published in Nature Neuroscience, examined microglia in extraordinary detail—mapping how their gene activity changes as Alzheimer’s advances.
The team studied brain tissue from 189 deceased individuals. Among them, 131 had been diagnosed with Alzheimer’s, representing a spectrum from mild to severe disease. The remaining 58 were cognitively healthy, showing no signs of dementia.
By isolating microglia from these samples and analyzing their RNA (the molecules that reflect which genes are active), researchers were able to create a kind of genetic fingerprint for microglia at different stages of Alzheimer’s. This technique, known as RNA sequencing, allowed them to see not only which genes were switched on or off but also how different versions of those genes (called isoforms) were used.
The results revealed something striking: microglia do not behave uniformly in Alzheimer’s disease. Instead, their gene activity shifts dramatically depending on disease severity and subtype. Some microglia seemed to adopt protective roles, while others appeared to worsen the damage.
The Hidden Diversity of Alzheimer’s
One of the most exciting findings from the Mount Sinai study is that Alzheimer’s may not be a single disease at the molecular level. Instead, it may exist in distinct subtypes, each with its own genetic signature.
Think of Alzheimer’s like a broad diagnosis of “fever.” A fever could be caused by a virus, a bacterial infection, or even an autoimmune condition—but each requires a different treatment. Similarly, Alzheimer’s may have multiple molecular paths leading to similar symptoms, which could explain why treatments that help some patients fail for others.
The researchers identified unique patterns of gene activity in microglia that were linked to dementia severity and the presence of hallmark brain lesions (like amyloid plaques and tau tangles). They also discovered that the coordination between certain genes broke down as the disease worsened, suggesting that Alzheimer’s disrupts not just individual genes but the networks connecting them.
This discovery is more than academic—it could reshape the way we approach treatment. If we can classify patients into subtypes based on microglial activity, we may be able to design personalized therapies that target the right molecular pathways for each individual.
Why This Matters for the Future
Right now, Alzheimer’s treatments mostly aim to slow cognitive decline by reducing amyloid plaques or modifying neurotransmitter activity. But these approaches have limited success. The Mount Sinai findings open an entirely new avenue: targeting microglia themselves.
If we can restore microglia to their protective state—or prevent them from tipping into harmful activity—we may be able to alter the course of the disease. This could be achieved through genetic therapies, immunological approaches, or drugs that fine-tune microglial function.
Imagine a future where doctors could test a patient’s microglial gene profile and prescribe a treatment tailored specifically to their Alzheimer’s subtype. It’s not science fiction; it’s a vision rooted in the discoveries unfolding right now in labs across the world.
The Emotional Weight of Discovery
Of course, scientific breakthroughs are not only about molecules and data. Behind every study are human stories—the families hoping for more time, the patients who donate their brains to research, the scientists who spend years chasing a tiny thread of possibility.
When researchers peer into a microscope or analyze genetic code, they are not just solving puzzles. They are working against the clock, racing to deliver hope to the millions of people who live each day in the shadow of Alzheimer’s.
For caregivers, the disease is an exhausting, heartbreaking journey. For patients, it is a gradual loss of independence and self. The promise of discoveries like this—that one day, Alzheimer’s may be prevented, slowed, or even reversed—carries enormous emotional weight.
A Glimpse Into Tomorrow
Science is a story, and with Alzheimer’s, that story is still being written. The chapter we are living through is one of transition: from treating symptoms to unraveling root causes. The Mount Sinai study marks a turning point, shining light on microglia as key players rather than bystanders.
The future may bring therapies that don’t just delay decline but reshape the disease itself. It may bring a world where a diagnosis of Alzheimer’s is not a sentence but a challenge that can be met with precision medicine.
Until then, each discovery—each insight into the brain’s immune system—brings us closer to that future. Microglia, once overlooked, may hold the key to restoring memory, identity, and dignity to millions.
And that is the hope that drives science forward: the belief that even in the face of one of humanity’s most devastating diseases, knowledge can bring light into the darkness.
More information: Roman Kosoy et al, Alzheimer’s disease transcriptional landscape in ex vivo human microglia, Nature Neuroscience (2025). DOI: 10.1038/s41593-025-02020-2