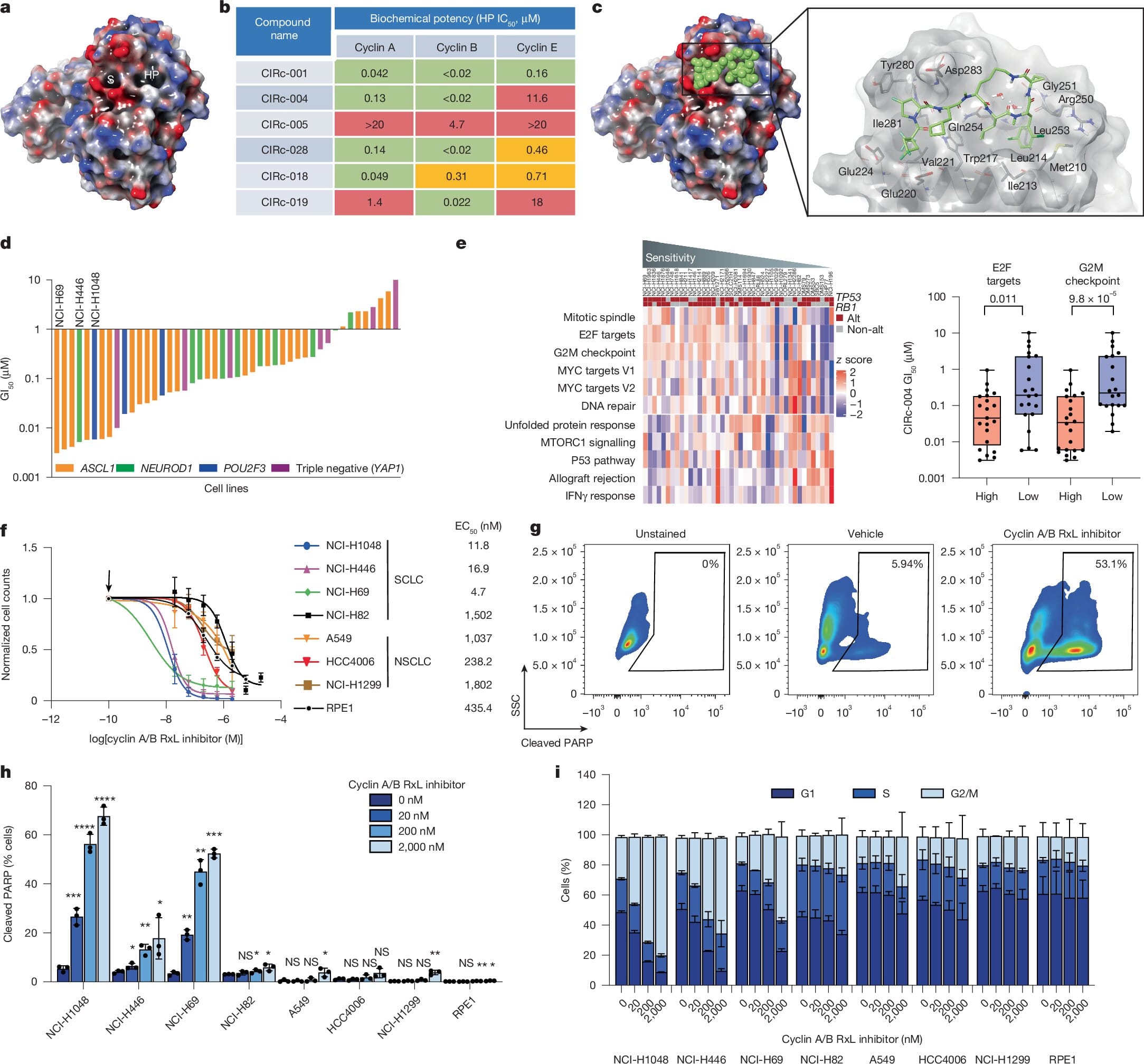
Small cell lung cancer (SCLC) is one of the most aggressive and deadly forms of cancer. Patients are often diagnosed at advanced stages, and while chemotherapy and immunotherapy can provide temporary relief, relapse is common and survival rates remain painfully low. For decades, researchers have searched for new ways to tackle this disease, but many attempts have been thwarted by the cancer’s biology.
Now, researchers at the Dana-Farber Cancer Institute have uncovered a breakthrough that could reshape treatment for SCLC and potentially other hard-to-treat cancers. Their work, published in Nature, demonstrates how a new class of drugs—direct cyclin inhibitors—can trigger a specific kind of cell death in cancer cells while leaving healthy cells largely unharmed. A phase 1 clinical trial is already underway nationwide, offering hope to patients with few options.
Cracking the Code of the Cell Cycle
At the heart of this discovery lies the cell cycle—the process by which cells grow and divide. In normal cells, this process is tightly controlled, with multiple checkpoints ensuring that damaged or unstable cells don’t progress to division. One of the most important of these checkpoints is the G1/S checkpoint.
Think of it as a quality-control gate: before a cell copies its DNA and moves toward division, it pauses at the G1/S checkpoint to check for errors. If something is wrong, proteins like RB1 and TP53 act as brakes, halting the process so repairs can be made.
But in cancers like small cell lung cancer, these brakes are often missing. Roughly 90% of SCLCs are driven by mutations that eliminate RB1 and TP53. Without them, cells rush recklessly through the checkpoint, accumulating errors that drive uncontrolled growth. For scientists, this has posed a major challenge. How do you target what isn’t there?
“It’s more like losing the brakes on a car than having something that is driving growth and can be blocked,” explains Dr. Matthew Oser, a thoracic oncologist and senior author of the new study.
An Old Idea, a New Possibility
The idea of targeting cyclins—the proteins that help control the cell cycle—is not new. In the late 1990s, Nobel Laureate Dr. William G. Kaelin Jr. proposed that cancers with disabled G1/S checkpoints could be uniquely vulnerable to cyclin inhibition. These cancers, marked by high activity of a factor called E2F, seemed to have an “Achilles’ heel.”
But for years, the chemistry required to make drugs that precisely block cyclin interactions proved elusive. That changed in the late 2010s, when Circle Pharma, a biotechnology company in San Francisco, developed new chemical approaches that made these drugs possible. The result was a class of molecules known as direct cyclin inhibitors.
How Direct Cyclin Inhibitors Work
In collaboration with Circle Pharma, the Oser Lab at Dana-Farber investigated exactly how these inhibitors act in cancer cells. Led by first author and postdoctoral fellow Shilpa Singh, the team discovered a two-step mechanism that explains why cancer cells die while normal cells survive.
The first step involves cyclin A. Normally, cyclin A interacts with E2F to regulate DNA replication. When the inhibitor blocks this interaction in cells that already have abnormally high E2F activity—such as cancer cells lacking RB1 and TP53—the result is excessive DNA damage.
The second step involves cyclin B. By blocking the interaction between cyclin B and another protein called MYT1, the drug prevents cells from properly navigating mitosis—the final stage of cell division. Without this safeguard, the cancer cell collapses during division.
Together, these two disruptions overwhelm the cancer cell’s already fragile control systems, leading to its death.
Why Normal Cells Are Spared
One of the most promising aspects of this therapy is its selectivity. Normal cells, which maintain balanced levels of E2F and intact checkpoints, do not suffer the same catastrophic DNA damage. According to Oser, “Normal cells are about 100- to 1,000-fold less sensitive to the drug than cancer cells. If the drug had had the same effect in normal cells, it would not be a feasible treatment.”
This dramatic difference means that researchers may be able to find a therapeutic dose that cripples tumors while sparing healthy tissue—something that has long been the holy grail of cancer therapy.
From the Lab to the Clinic
The Oser Lab tested the drug in patient-derived xenografts—models in which human tumor tissue is implanted into mice. In these experiments, small cell lung cancer tumors treated with the drug stopped growing, demonstrating clear anti-cancer activity. Additional studies suggested the drug could also work against other cancers that have lost G1/S checkpoint control, including triple-negative breast cancer, another highly aggressive disease.
Encouraged by these results, Dana-Farber and partners have launched a phase 1 clinical trial across the United States. The trial will test a related compound called CID-078 in patients with small cell lung cancer, triple-negative breast cancer, and other cancers with similar vulnerabilities.
For patients and doctors, this trial represents more than just another study—it is a chance to test a fundamentally new therapeutic strategy in diseases that desperately need new options.
A Turning Point in Cancer Medicine
The journey from basic science to patient care is long and often uncertain. But the story of direct cyclin inhibitors shows how ideas can persist through decades of research, waiting for the right scientific tools to make them reality. From Kaelin’s hypothesis in the 1990s, to breakthroughs in chemistry in the 2010s, to the Oser Lab’s mechanistic discoveries today, this progress is a testament to persistence and collaboration.
If successful, this approach could redefine how scientists think about treating cancers with missing tumor suppressors—shifting the focus from what is present to what is absent.
Looking Ahead
The next few years will be critical. Phase 1 trials are designed to test safety and dosing, not yet to prove efficacy. But if CID-078 continues to demonstrate strong selectivity for cancer cells, it could pave the way for larger studies and, ultimately, new approved treatments.
For patients facing small cell lung cancer or triple-negative breast cancer, the news brings hope where options have long been limited. And for the broader field of oncology, it signals the arrival of a powerful new strategy: directly targeting the cell cycle’s most critical engines.
As Dr. Oser reflects, “Our research using cell biology and genetic screening reveals a two-step mechanism of cell death specifically in cancer cells that does not occur in normal cells.” That insight may be the key to tipping the balance against some of the deadliest cancers known today.
More information: Shilpa Singh et al, Targeting G1–S-checkpoint-compromised cancers with cyclin A/B RxL inhibitors, Nature (2025). DOI: 10.1038/s41586-025-09433-w