For decades, scientists believed that the epithelial cells lining the cervix were little more than a passive shield—a wall separating the external environment from the body’s inner sanctum. They were thought to form a barrier, yes, but not to participate in the battle raging between invading pathogens and the immune system.
Now, that view has been turned on its head. In a groundbreaking study published in Science Advances, an international research team led by Associate Professor Cindrilla Chumduri from Aarhus University has unveiled a far more dynamic picture. The cervix, it turns out, is not merely a gateway—it’s an active, intelligent fortress. Its epithelial cells don’t just stand guard; they communicate, coordinate, and fight.
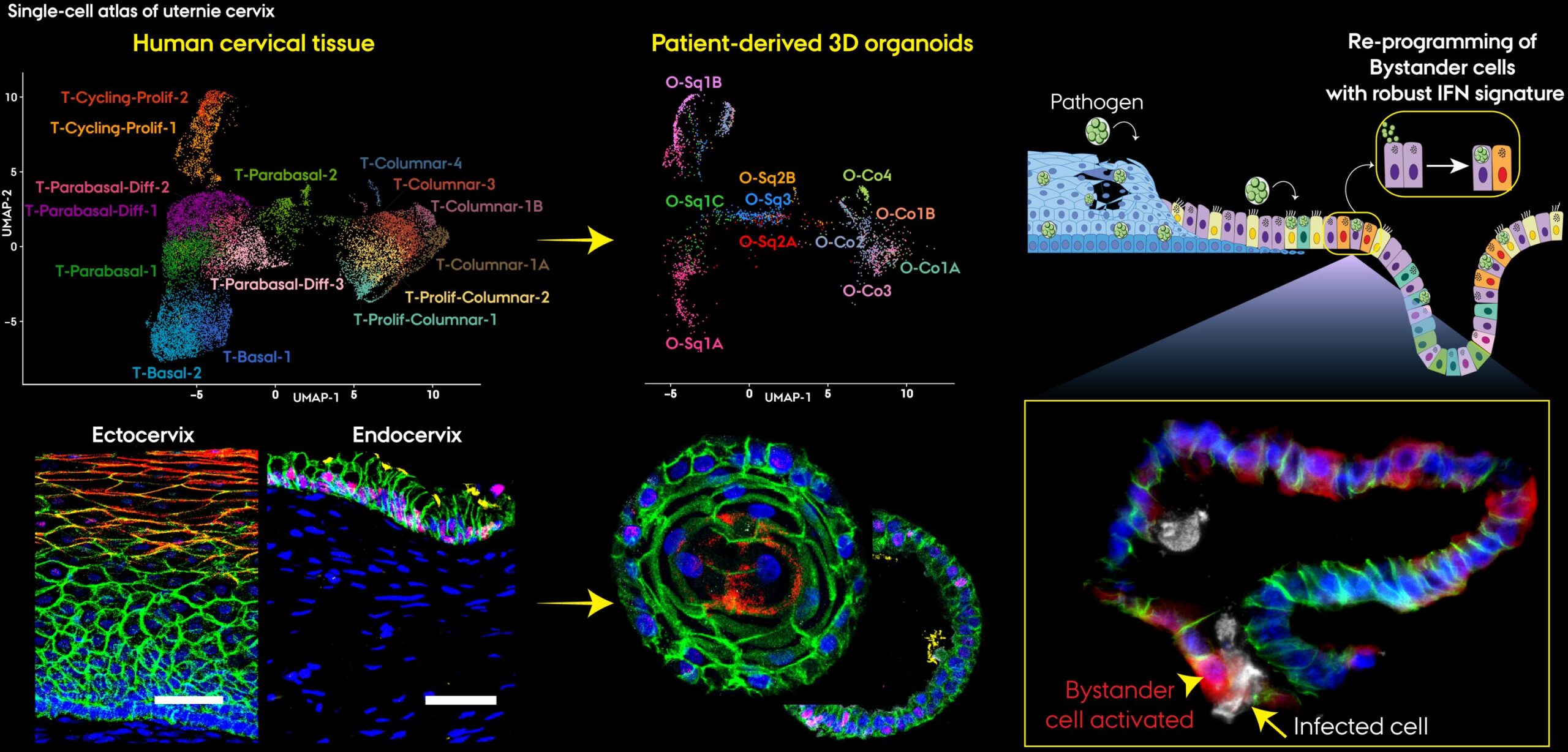
Using cutting-edge tools such as patient-derived 3D organoids—tiny, lab-grown versions of human cervical tissue—and single-cell mapping technologies, the researchers discovered that the cervical epithelium orchestrates an intricate immune defense. Each cell type plays a unique and purposeful role, forming a multilayered network capable of detecting, signaling, and neutralizing infection.
This revelation doesn’t just rewrite textbooks; it transforms how we understand one of the body’s most vital and misunderstood organs.
The Living Architecture of Defense
The cervix sits at a critical intersection between the uterus and the vagina, acting as both gateway and guard. It consists of two distinct regions: the ectocervix, lined with protective squamous epithelial cells, and the endocervix, lined with more delicate columnar cells that secrete mucus and interact with immune molecules.
These layers were once seen as static structures. But Chumduri’s team has shown that they are, in fact, alive with activity—an ecosystem of specialized cells constantly sensing their environment and coordinating defense strategies.
Using organoids, the researchers recreated cervical tissue in three dimensions. Unlike flat cell cultures used in traditional laboratory experiments, organoids mimic the architecture and behavior of real tissue. Each cell retains its unique identity, function, and interaction patterns. This allowed the scientists to observe how different epithelial subtypes behave under attack, particularly when faced with Chlamydia trachomatis, the world’s most common bacterial sexually transmitted infection.
When Cells Begin to Speak
One of the most striking discoveries was that cervical cells communicate—not with words, of course, but with chemical messages. Using single-cell RNA sequencing, the team could read the genetic “conversation” of thousands of individual cells. Each message, each molecule, told part of a story: who was calling for help, who was repairing, who was amplifying the immune alarm.
The outer ectocervical cells strengthened their defenses, reinforcing the barrier against invasion. Meanwhile, deeper endocervical cells switched on interferon pathways, producing antiviral and antibacterial signals. Some began presenting antigens—the biological equivalent of raising a red flag to alert the immune system to danger.
And yet, the most surprising revelation was this: the strongest defenders were not the ones infected.
The Uninfected Heroes
“The most striking finding was that uninfected cells became the most immunologically active,” explains Pon Ganish Prakash, the study’s first author. These healthy neighboring cells sensed distress signals from infected ones and immediately mobilized their own defenses.
This discovery paints a breathtaking picture of biological cooperation. The cervix isn’t just reacting randomly to infection—it’s strategizing. When invaders breach the outermost layer, deeper epithelial cells leap into action, activating immune genes even without direct infection. It’s as if a community of sentinels were standing on the ramparts, lighting torches to warn others before the enemy reaches the gates.
Such communication is critical. If only infected cells responded, the defense would be too late. By sharing information across cell types, the cervix creates a rapid, coordinated, and highly localized immune response—powerful enough to stop pathogens before they spread upward into the uterus and fallopian tubes.
A Symphony of Signals
Beyond identifying which cells responded, the researchers also uncovered how they coordinated. Their analysis revealed a web of cellular “conversations,” where certain subtypes acted as hubs—conductors in a biological orchestra.
Some epithelial cells specialized in repair, producing growth factors that helped heal tissue damaged during infection. Others focused on amplifying immune signals, ensuring that defensive molecules such as cytokines and interferons were released in the right amounts at the right time.
This balance between defense and healing is crucial. Too much inflammation can scar tissue and cause fertility problems; too little, and infections can linger or spread. The cervical epithelium has evolved to walk that delicate line with exquisite precision, guided by communication between its diverse cell types.
Associate Professor Chumduri summarizes it beautifully: “The cervix is not just a physical barrier but a truly immunocompetent tissue that orchestrates complex defense mechanisms.”
Chlamydia: A Global Challenge
The team’s focus on Chlamydia trachomatis underscores the urgent relevance of their findings. Chlamydia infects millions of people each year, often silently. Left untreated, it can cause pelvic inflammatory disease, infertility, ectopic pregnancies, and increase susceptibility to other infections, including HIV.
By mapping how cervical cells detect and respond to chlamydia, scientists can begin to identify weak spots in the infection cycle—and potential new strategies to strengthen natural immunity.
The hope is that this deeper understanding of mucosal immunity will lead to better vaccines and therapies that activate the body’s defenses right where they’re needed most: at the infection site.
Toward a New Generation of Mucosal Vaccines
Traditional vaccines are injected into the bloodstream, training the systemic immune system to recognize and attack pathogens. But sexually transmitted infections often take hold at mucosal surfaces—the moist tissues of the reproductive, respiratory, or digestive tracts—where systemic immunity may not reach effectively.
That’s why Chumduri and her colleagues see enormous promise in mucosal vaccines. By targeting the local epithelial and immune cells in the cervix, such vaccines could provide frontline protection before an infection even begins.
“We see huge potential in developing mucosal vaccines that can activate local immunity right at the infection site,” Chumduri explains. Such an approach could revolutionize prevention of STIs like chlamydia and HPV, reducing global disease burdens and their long-term consequences, including cervical cancer.
Mini-Organs, Major Breakthroughs
At the heart of this research lies an extraordinary technology: organoids. These miniature, lab-grown versions of human tissues are created from patient cells and can replicate the structure, diversity, and function of real organs.
“Mini-organs are like a window into human tissue,” says Postdoc Naveen Kumar Nirchal, a co-author of the study. “They allow us to test ideas and treatments in highly realistic models, far more accurate than traditional lab methods.”
Unlike animal models or flat cell cultures, organoids can mimic how human cells behave in a living body. This makes them ideal for studying infections, testing drugs, and understanding how diseases unfold over time. In the case of the cervix, organoids reveal not only how infections begin but how tissues heal, communicate, and adapt.
These 3D models could also help researchers explore co-infections—for instance, how HPV and chlamydia might interact to increase the risk of cancer. Understanding such relationships could guide more effective prevention and treatment strategies in the future.
Cellular Diversity: The Secret Strength
A key insight from the study is that not all epithelial cells are alike, even within the same tissue. The cervix’s defense depends on this diversity—what scientists call epithelial heterogeneity.
“Each subtype has its own job in protecting the cervix and preventing the spread of infections to upper reproductive organs,” explains Senior Researcher Rajendra Kumar Gurumurthy.
Some cells act as front-line defenders, others as messengers, others as healers. It’s a division of labor that mirrors the complexity of the immune system itself. This discovery may also change how we view other mucosal tissues, such as those in the gut, lungs, and urinary tract, which share similar layered architectures.
Understanding these hidden differences could lead to more precise medical treatments—targeting not just an infection, but the specific cell types that interact with it.
Beyond Infection: A Platform for Regeneration and Cancer Research
The implications of this work go beyond fighting infections. Because cervical organoids closely replicate native human tissue at single-cell resolution, they provide a powerful platform for studying cancer, regeneration, and cellular repair.
Researchers can now observe how precancerous changes develop, how cells lose their normal communication patterns, and how healthy tissue might be coaxed into repairing itself. In the long run, this could lead to advances in regenerative medicine—helping the body heal damaged tissues by stimulating its own cellular intelligence.
In the same way that this study revealed hidden roles for cervical cells in immunity, future research may uncover similar hidden potentials in other tissues. The line between immune system and structural tissue, once considered sharp, is growing blurrier—and that is a good thing.
The Global Stakes
Sexually transmitted infections remain a massive public health challenge. More than one billion people worldwide are affected by STIs each year. Many of these infections go undiagnosed and untreated, leading to serious long-term complications including infertility, chronic pain, and cancer.
This new understanding of cervical defense is more than a scientific breakthrough—it’s a beacon of hope. By unlocking the body’s own protective systems, researchers can design interventions that are more natural, more precise, and more sustainable.
If local immunity can be enhanced, if vaccines can work where pathogens first enter, then the burden of STIs could be drastically reduced. Millions of women worldwide could be spared the silent suffering that infections often cause.
Redefining the Body’s Boundaries
This research also challenges one of biology’s oldest assumptions—that immune defense is the sole domain of specialized immune cells like macrophages or T cells. The cervix tells a different story. Its epithelial cells, once dismissed as passive bystanders, are revealed as intelligent participants, capable of sensing, signaling, and responding in real time.
In essence, the boundary between self and defense is not as clear as we once thought. Every cell, it seems, carries a piece of the immune system’s intelligence. This revelation transforms how we understand not just the cervix, but the entire human body—as a network of cooperating tissues, each contributing to our survival.
The Road Ahead
Science often advances in quiet revolutions. This discovery—of active, communicative, immune-capable epithelial cells—may well be one of them. It bridges disciplines: immunology, microbiology, reproductive health, and systems biology. It invites collaboration between engineers, clinicians, and biologists.
For the researchers at Aarhus University and their collaborators, the next steps are already underway. They are exploring how hormones, microbiota, and environmental factors influence cervical immunity, and how this knowledge can be translated into real-world therapies.
Their work reminds us that the body’s most overlooked structures often hold the greatest secrets.
A Living Fortress
The cervix is no longer just an anatomical passage—it is a living fortress, a sophisticated sentient barrier that defends, heals, and adapts. Every moment, millions of epithelial cells perform their quiet duty, keeping pathogens at bay while maintaining the delicate balance needed for fertility and reproduction.
This discovery doesn’t merely deepen our understanding of one tissue—it transforms our vision of human biology itself. The human body is not a battlefield where the immune system fights alone; it is a symphony, where every instrument plays its part in maintaining harmony.
And within that symphony, the cervix—long overlooked, long misunderstood—emerges as a conductor in its own right.
Through the lens of modern science, we can finally see what was always there: a living, breathing shield at the heart of womanhood, defending life in silence, sophistication, and strength.
More information: Pon Ganish Prakash et al, Single-cell atlas of cervical organoids uncovers epithelial immune heterogeneity and intercellular crosstalk during Chlamydia infection, Science Advances (2025). DOI: 10.1126/sciadv.ady1640. www.science.org/doi/10.1126/sciadv.ady1640