Few scientific discoveries touch both the intellect and imagination as powerfully as those that hint at regeneration—the ability to rebuild what nature has taken away. For centuries, the idea of regrowing lost teeth has hovered somewhere between myth and miracle. But now, an extraordinary breakthrough from a team at the Institute of Science Tokyo and their collaborators around the world has brought that vision closer to reality.
In two companion papers published in Nature Communications on July 1 and July 2, 2025, researchers revealed the discovery of two distinct stem cell lineages responsible for forming the tooth root and the alveolar bone—the jawbone structure that cradles each tooth. Using cutting-edge genetic tracing techniques in mice, they have illuminated how specialized cell populations coordinate to sculpt one of nature’s most complex and resilient biological architectures: the tooth and its surrounding bone.
This discovery is not merely an incremental advance; it represents a leap forward in our understanding of dental biology, offering new hope for future regenerative therapies that could one day allow humans to naturally regrow lost teeth.
The Quest to Recreate Nature’s Design
For decades, dentistry has relied on mechanical replacements—implants, bridges, and dentures—to restore lost teeth. While these solutions are practical and life-changing for millions, they are, at best, imitations of nature. A real tooth is a living structure, with nerves, blood vessels, and a delicate dance of tissues that grow and adapt over time.
Regenerating a lost tooth, along with its supporting bone, is considered the “holy grail” of dental medicine. To achieve it, scientists must not only recreate a tooth’s outer form but also replicate the intricate relationships between its internal tissues and the bone that holds it in place. Yet the biological processes behind tooth and bone development remain a labyrinth of mysteries.
Teeth do not grow in isolation. Their formation involves an orchestra of tissues—the dental pulp, enamel organ, dental follicle, and alveolar bone—all communicating through a symphony of molecular signals. Each cell must “know” what to become, when to divide, and when to stop. One misstep in this choreography can disrupt the entire structure.
Understanding this interplay at the cellular level has been one of the great challenges of developmental biology. That is precisely where the Science Tokyo team stepped in.
Peering into the Heart of Development
The research, led by Assistant Professor Mizuki Nagata of the Department of Periodontology at Science Tokyo, and Dr. Wanida Ono from the University of Texas Health Science Center at Houston, represents a remarkable international collaboration with partners from the University of Michigan and other leading institutions.
Their goal was simple but ambitious: to map, in exquisite detail, how stem cells in developing teeth decide their fate—whether to become part of the tooth itself or the bone that anchors it.
To accomplish this, the scientists turned to genetically modified mice and lineage-tracing techniques—powerful methods that allow researchers to follow the destiny of individual cells over time. Using fluorescent tags that glow under specific lighting conditions, they could literally watch cells transform, migrate, and organize themselves during tooth growth.
By silencing specific genes and manipulating key signaling pathways, they uncovered how different molecular “instructions” guide these stem cells into specialized roles. What they found fundamentally redefines how we understand tooth development.
Two Lineages, One Beautiful Collaboration
The study revealed two distinct, previously unrecognized populations of mesenchymal stem cells—the master builders responsible for creating the supportive structures of the tooth and jaw.
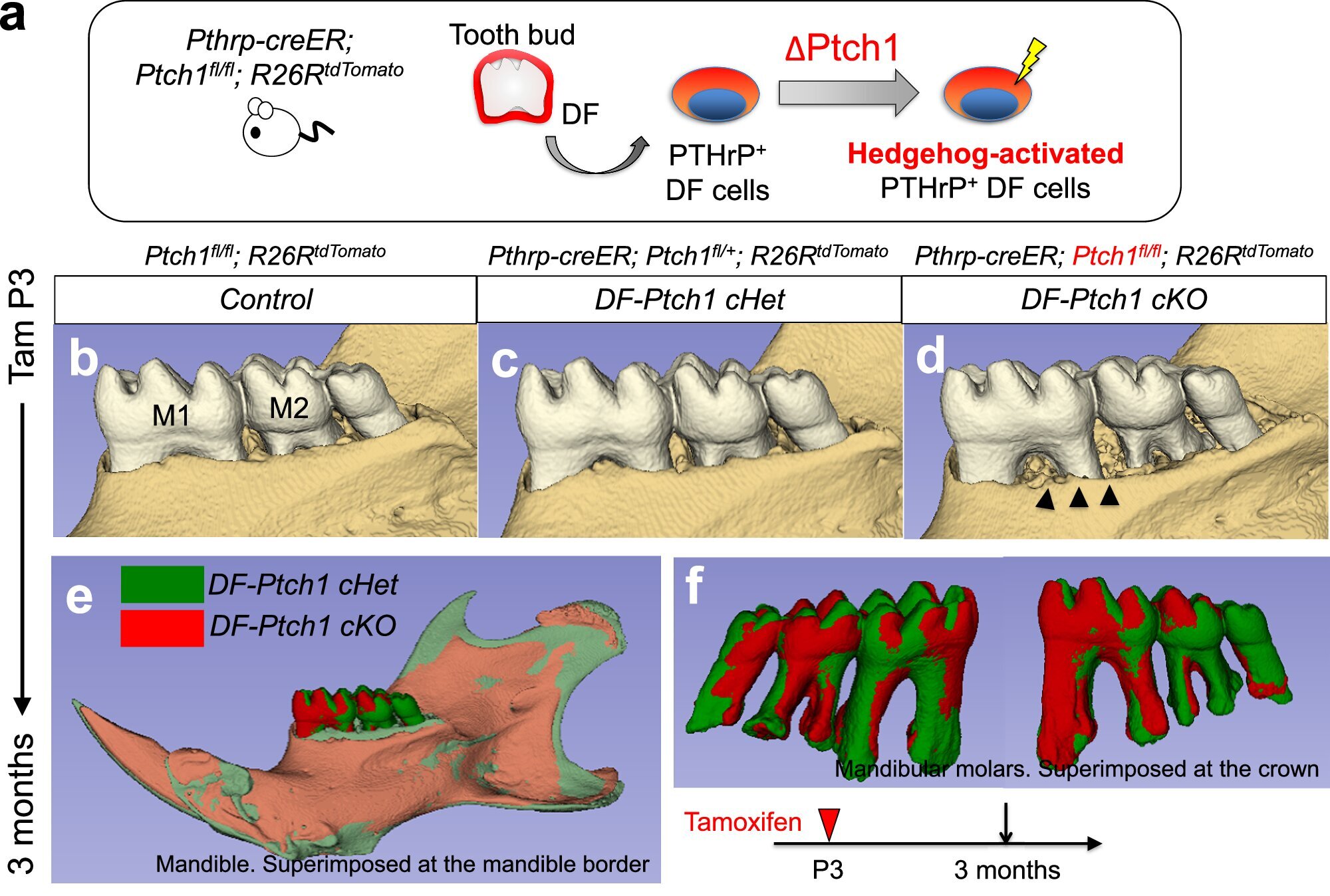
The first lineage was traced to the apical papilla, a soft tissue mass located at the tip of a growing tooth root. These cells, marked by the presence of a signaling protein called CXCL12, play a crucial role in the formation of bone within the bone marrow. Through the canonical Wnt pathway—a key communication channel in stem cell biology—these CXCL12-expressing cells can differentiate into several types of cells vital for tooth and bone formation.
Remarkably, under the right conditions, these cells can transform not only into odontoblasts, which form dentin (the hard tissue beneath enamel), but also into cementoblasts, which build the cementum covering the tooth root, and even into osteoblasts, which generate the alveolar bone itself.
The second lineage was found within the dental follicle, a sac-like structure surrounding the developing tooth. This population of cells expressed parathyroid hormone-related protein (PTHrP)—a molecule known for regulating bone metabolism. These cells, too, showed a remarkable versatility, capable of becoming cementoblasts, ligament-forming fibroblasts, and alveolar bone osteoblasts.
However, this transformation depended on a very particular molecular balance. The researchers discovered that a signaling pathway known as the Hedgehog–Foxf pathway had to be switched off for the PTHrP-expressing cells to fully commit to forming bone. It was as if nature had installed a safety switch, preventing premature or misdirected bone formation unless all conditions were just right.
“We observed that the Hedgehog–Foxf pathway needs to be suppressed to drive the alveolar bone osteoblast fate of PTHrP-expressing cells,” explains Nagata. “This reveals a unique tooth-specific mechanism of bone formation that requires deliberate on–off regulation of Hedgehog signaling.”
This discovery paints a stunning picture: two distinct stem cell lineages, each with its own developmental logic, working in harmony to construct both the tooth root and its surrounding support structure.
The Language of Cells: How Signals Shape Identity
One of the most captivating aspects of this research lies in the molecular dialogue between cells. Stem cells are not pre-programmed to become a particular type of tissue; instead, they respond to the chemical signals in their environment—tiny messages carried by proteins and other molecules.
In tooth development, these signals form a complex web. The Wnt pathway, for instance, acts like a conductor guiding stem cells to differentiate in specific directions. When Wnt signaling is activated, stem cells are nudged toward forming the hard, mineralized tissues of the tooth root. The Hedgehog pathway, on the other hand, operates as a kind of brake—restraining certain transformations until the surrounding structures are ready.
It is this dynamic push and pull—activation and inhibition—that allows the delicate architecture of a tooth to form correctly. By decoding these instructions, scientists gain not only a deeper understanding of natural development but also a toolkit for future regenerative strategies.
Building the Foundation for Regenerative Dentistry
The implications of these findings stretch far beyond basic biology. They represent a turning point for regenerative dental medicine, a field that seeks to replace lost or damaged tissues with living, functional ones.
If researchers can learn to harness these stem cell populations, it may one day be possible to grow new teeth from a patient’s own cells—teeth that integrate naturally with the surrounding bone and nerves, eliminating the need for artificial implants. Even partial regeneration—such as rebuilding the tooth root or strengthening the jawbone—could transform dental care for millions worldwide.
Furthermore, these insights could help repair periodontal disease, a major global cause of tooth loss. By guiding stem cells to regenerate the cementum and bone destroyed by infection or inflammation, doctors could restore not just appearance but true biological function.
“This mechanistic framework for tooth root formation gives us a roadmap,” says Nagata. “It opens the way for innovative stem-cell-based regenerative therapies for dental pulp, periodontal tissues, and bone.”
The Art and Emotion of Scientific Discovery
Beyond its medical promise, this research also embodies something deeply human—the desire to understand and recreate the beauty of nature’s designs. The tooth, often overlooked as a simple piece of anatomy, is in fact a masterpiece of biological engineering. It must endure decades of wear and stress, yet remain sensitive and responsive to the world around it.
For Nagata and her colleagues, uncovering the hidden rules of how a tooth builds itself was not merely an intellectual puzzle—it was a journey into life’s most elegant processes. Watching fluorescent cells bloom across a microscope’s field, mapping their migrations and transformations, is to witness creation in miniature.
There is something profoundly poetic in the fact that our understanding of regeneration—of renewal and healing—begins with something as humble as a tooth. It reminds us that even the smallest parts of our bodies hold vast mysteries, and that in decoding them, we learn not only how to rebuild our tissues, but how to appreciate the astonishing complexity of life itself.
A Future of Living Dentistry
The road ahead is long, and many challenges remain before these discoveries can translate into clinical treatments for humans. Scientists will need to determine how to safely manipulate similar pathways in human cells, how to control differentiation with precision, and how to integrate newly grown tissues seamlessly into existing bone structures.
Yet, the foundations are now set. The identification of these two stem cell lineages offers a blueprint—a cellular map of how nature constructs the intricate interface between tooth and bone. The regenerative dentistry of the future may no longer rely on titanium or ceramic, but on the body’s own biological power to heal and rebuild.
Imagine a world where a lost tooth does not mean a permanent gap or an artificial implant, but simply the beginning of a new growth cycle—where dental visits focus on activating your body’s own regenerative potential. That world, once confined to the realm of dreams, is now coming into view.
More information: Mizuki Nagata et al, Wnt-directed CXCL12-expressing apical papilla progenitor cells drive tooth root formation, Nature Communications (2025). DOI: 10.1038/s41467-025-61048-x
Mizuki Nagata et al, A Hedgehog–Foxf axis coordinates dental follicle-derived alveolar bone formation, Nature Communications (2025). DOI: 10.1038/s41467-025-61050-3