In a world where opioid overdose has claimed more than 80,000 lives in a single year, the discovery of a new kind of pain reliever could mark a historic turning point. Researchers at Kyoto University have unveiled ADRIANA, a groundbreaking non-opioid analgesic that may offer the same pain-relieving benefits as morphine or fentanyl—without the devastating side effects of respiratory depression or drug dependence.
As the opioid crisis continues to devastate families and communities, particularly in the United States, where synthetic opioids like fentanyl have fueled a full-blown public health emergency, the need for safer, more effective pain treatments has never been more urgent. ADRIANA, if successful, could be the first real alternative in decades—a molecule that promises pain relief without addiction.
The Science Behind the Discovery
Pain, as debilitating as it is universal, is typically managed using opioid drugs that act on receptors in the brain to dull perception. However, opioids come at a steep cost. They flood the reward system with dopamine, making the user feel not only relief but euphoria. Over time, this effect can rewire the brain, leading to physical dependence, tolerance, and withdrawal—hallmarks of addiction.
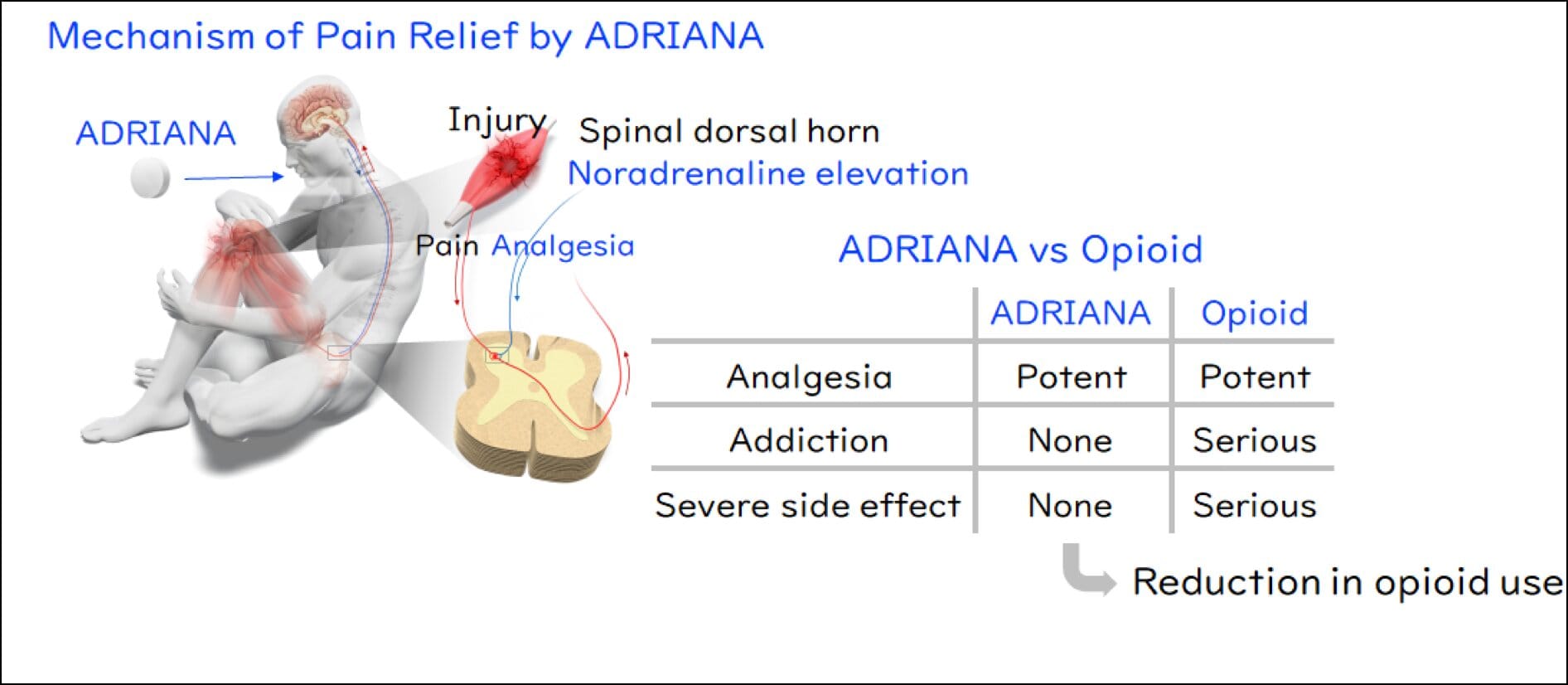
But the Kyoto University team took a radically different approach. Instead of mimicking the opioid pathway, they turned their attention to noradrenaline, a naturally occurring chemical released by the body in times of stress or danger. Noradrenaline activates receptors in the nervous system known as α2-adrenoceptors, which play a crucial role in modulating pain.
Previous studies had explored substances that mimic noradrenaline to activate these receptors—especially α2A-adrenoceptors, which are known to inhibit pain signals. However, those substances also activated α2B-adrenoceptors, which can dangerously destabilize cardiovascular function. This balancing act between pain relief and safety remained unresolved—until now.
By analyzing the behavior of noradrenaline and these receptor subtypes, the research team proposed a counterintuitive idea: What if they blocked α2B-adrenoceptors instead? This, they hypothesized, would cause the body to produce more noradrenaline, naturally activating α2A receptors and relieving pain—without the cardiovascular side effects.
To test this, the team deployed a cutting-edge molecular screening technique called the TGFα shedding assay. This method allowed them to precisely measure how chemical compounds interact with individual receptor subtypes. After screening a vast library of compounds, they made a historic breakthrough: the world’s first selective α2B-adrenoceptor antagonist.
From Mice to Medicine: Promising Early Results
The next step was to prove that this discovery could translate into meaningful treatment. In mice, the new compound reduced pain behaviors without signs of cardiovascular distress. Importantly, it also avoided the euphoric brain effects typical of opioids—a key indicator of its low addiction potential.
Following promising results in preclinical safety studies, physician-led trials were conducted at Kyoto University Hospital. A Phase I trial with healthy human volunteers showed that the drug was well tolerated. Then came the more challenging Phase II trial, involving patients recovering from painful lung cancer surgeries. Again, the outcomes were remarkable: patients experienced significant pain relief with no respiratory depression or cardiovascular instability.
These results, while early, suggest ADRIANA could deliver pain relief on par with opioids—but without the chains of dependency.
A Global Effort for a Global Crisis
Now, with the backing of BTB Therapeutics, Inc., a biotech company spun out from Kyoto University, plans are underway for a large-scale Phase II trial in the United States. This effort represents not only a triumph of Japanese science but a rare and much-needed gesture of international collaboration in the face of a shared crisis.
In the U.S., where decades of overprescription of opioid painkillers like OxyContin have fueled widespread addiction and social collapse in some communities, the need for innovation is not just scientific—it is moral. If ADRIANA succeeds, it could help rewrite clinical guidelines, reduce reliance on opioids in post-surgical care and chronic pain management, and ultimately save thousands of lives.
“This is more than just a drug discovery,” says Masatoshi Hagiwara, the study’s senior author and a specially-appointed professor at Kyoto University. “It’s a new philosophy of pain management.”
What Sets ADRIANA Apart
What makes ADRIANA truly revolutionary is its mechanism of action. Unlike opioids, which directly stimulate reward centers in the brain and carry a high potential for misuse, ADRIANA works by leveraging the body’s natural pain modulation system—essentially tricking the nervous system into releasing its own painkillers without risking addiction.
By blocking α2B-adrenoceptors, the drug encourages noradrenaline to accumulate and selectively engage α2A-adrenoceptors, delivering targeted pain relief. Because it does not act on opioid receptors, it avoids the respiratory depression and euphoric high associated with traditional narcotics. This dual effect—high efficacy with low risk—is what makes ADRIANA a potential game-changer.
It could also prove beneficial for patients who cannot tolerate opioids due to existing heart or lung conditions, or those in recovery from substance use disorders who need non-addictive options for pain relief.
Japan’s Strict Drug Policy Shaping Innovation
Japan is known for its cautious approach to opioid use. Medications like morphine and fentanyl are strictly regulated and prescribed only under close supervision by authorized physicians. This cultural and regulatory environment likely influenced Kyoto University’s direction in pharmaceutical research, fostering an urgency to find alternatives that are effective but carry fewer societal risks.
The restrictive landscape around opioids in Japan, rather than stifling innovation, may have driven it. With fewer options available domestically, researchers were motivated to pursue bold alternatives—and succeeded.
Looking Ahead: The Long Road to Approval
Despite the excitement, there are still many hurdles before ADRIANA can reach pharmacies and hospitals around the world. The upcoming U.S. trial will need to demonstrate efficacy across a broader range of pain types, including chronic conditions like fibromyalgia, arthritis, and neuropathic pain. Long-term safety data will also be crucial, as will assessments of how ADRIANA interacts with other common medications.
But optimism is high. If ADRIANA performs as expected in larger trials, it could receive FDA Fast Track designation, which would accelerate the review process. Commercial partnerships and global licensing deals may follow, positioning Kyoto University—and Japan—as leaders in the next generation of pain science.
“We envision a future where pain can be treated without fear,” says Hagiwara. “Where patients are not forced to choose between suffering and addiction.”
A New Chapter in Medical Science
As nations continue to battle the opioid epidemic, the emergence of ADRIANA marks a possible new chapter—not just in pharmacology, but in how societies understand and treat pain. It represents a quiet revolution born not from corporate labs, but from academic insight, careful experimentation, and a profound desire to do better for humanity.
Science, at its best, does not only illuminate how the world works—it heals. If ADRIANA continues to prove itself, it could become one of the great healing stories of our time.
For now, the world watches—and hopes.
More information: Hagiwara, Masatoshi, Discovery and development of an oral analgesic targeting the α2B adrenoceptor, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2500006122. doi.org/10.1073/pnas.2500006122