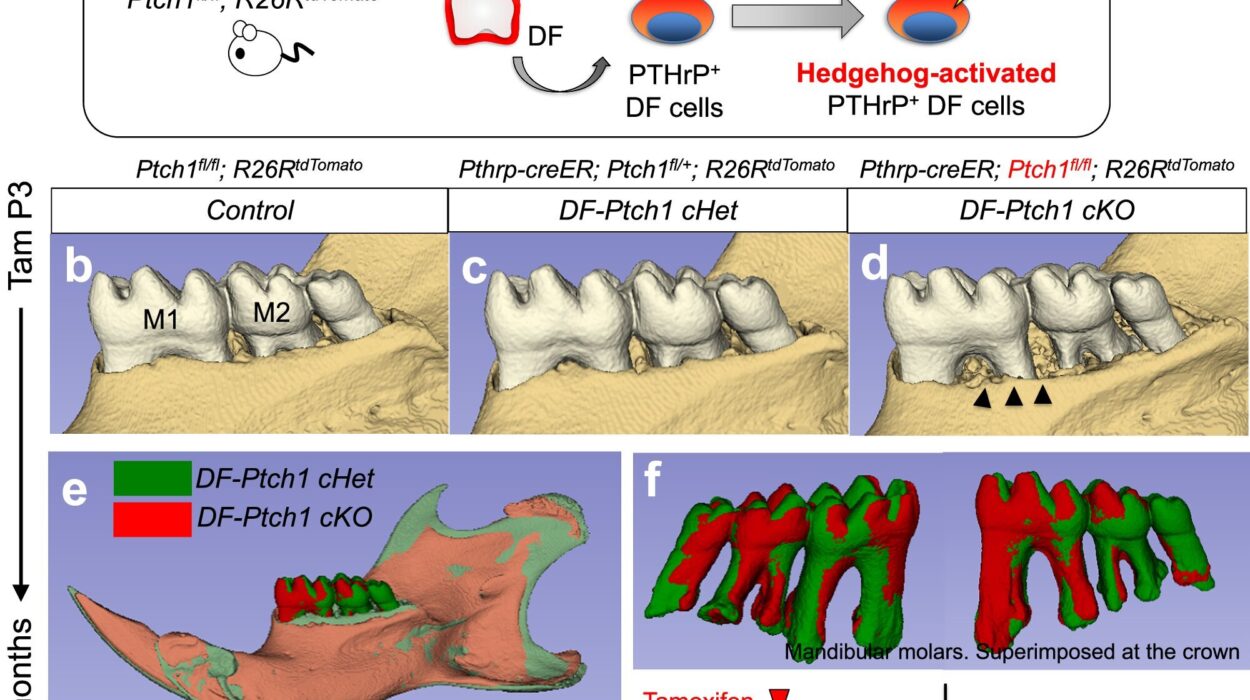
For countless patients across the world, daily injections are a painful but unavoidable part of life. From children needing growth hormone therapy to adults managing chronic conditions like diabetes or undergoing cancer immunotherapy, the sharp sting of a needle is the daily price of survival. But a new breakthrough from researchers at the University of Bath may soon rewrite this medical reality—and bring the world a step closer to a future without injections.
Led by Professor Randy Mrsny from the University of Bath’s Department of Life Sciences, the research team has developed a pioneering method that enables protein-based drugs—previously only injectable—to be taken orally as a pill. The study, recently published in the Journal of Controlled Release, marks a major step forward in the long-standing quest to make powerful therapeutic proteins as easy to take as a common aspirin.
Why Protein-Based Drugs Can’t Be Taken as Pills—Until Now
Unlike traditional pills for ailments like headaches or infections, drugs made of proteins—such as insulin, antibodies, or human growth hormone—can’t survive the harsh environment of the human stomach. When swallowed, they are rapidly broken down by stomach acid and digestive enzymes before they ever have a chance to enter the bloodstream.
This is why treatments involving proteins, like diabetes drugs such as Ozempic and Wegovy, or immunotherapy for cancer, must be injected. These injections are often painful, expensive, and inconvenient. Over time, they can lead to missed doses, particularly in patients who require lifelong treatment. For children or individuals with needle phobia, this daily ritual can be emotionally distressing.
The new technology developed at the University of Bath may finally offer a solution—a way to sneak these fragile therapeutic proteins past the digestive gauntlet and into the bloodstream safely and effectively.
A Bacterial Secret Turned Into a Medical Solution
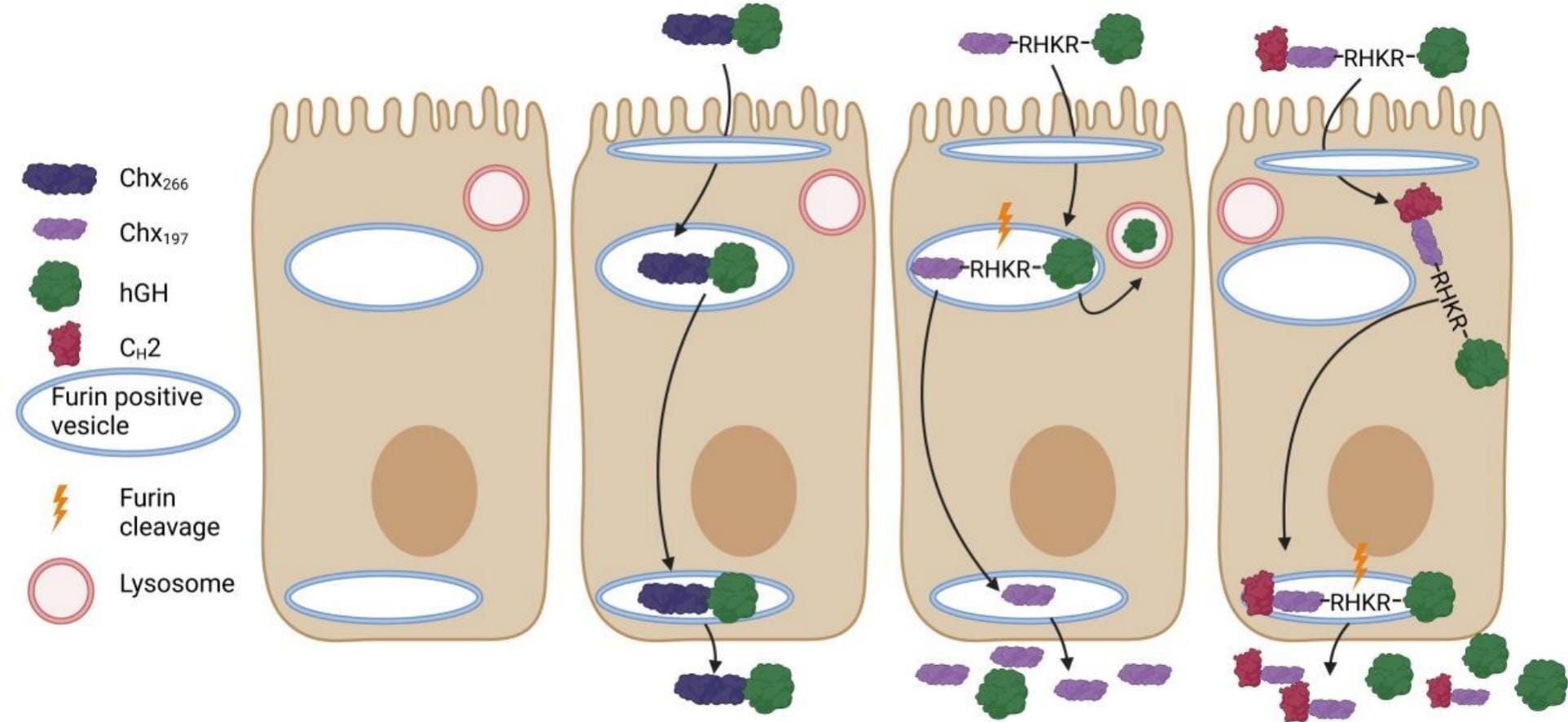
The Bath research team found inspiration not in high-tech materials or robotics, but in nature—specifically, in the clever strategies used by bacteria in the human gut. Some bacteria have evolved specialized systems to transport molecules across the intestinal wall, and one of these was co-opted by the scientists to help deliver drugs instead of disease.
In their study, the team linked a drug molecule—human growth hormone in this case—to a harmless carrier protein derived from a bacterium related to cholera. Importantly, this bacterial component is non-toxic and repurposed solely to exploit its unique ability to interact with a specific receptor found on the cells lining the human intestine.
This receptor, normally used by the bacterium to gain entry into the body, acts like a molecular doorway. Once the drug-carrier complex binds to the receptor, it is transported across the intestinal lining and released into the bloodstream intact.
Reliable, Safe, and Effective
One of the most promising features of this new system is its consistency. In lab tests involving rats, the delivery platform was able to transport 5–10% of the administered drug dose into the bloodstream. While this might sound small, it’s a clinically significant level for many powerful protein-based therapies.
Crucially, the system doesn’t cause damage to the intestinal wall—a major issue with previous oral delivery technologies. Many past attempts to create “oral biologics” (drugs made from biological sources) have failed because they either triggered inflammation, damaged cells, or were simply too unreliable in how much drug was absorbed.
Professor Mrsny emphasized that this new approach is different: “While it’s not the first system to replace injections, ours is the first platform to work safely and consistently, delivering the drug at effective doses and using a well-understood pathway.”
What Happens Next?
Following their successful trials in rats, the Bath team is now collaborating with pharmaceutical companies to fine-tune the delivery platform and adapt it for a wider range of drugs. If all goes well, human clinical trials could begin within the next two years.
“We know the pathway works in humans because it’s based on natural processes in the human intestine,” explained Professor Mrsny. “That gives us a high degree of confidence that what we’ve seen in animal models will translate to people.”
What’s even more exciting is the versatility of the method. Unlike earlier systems, which had to be tailored for specific drugs, this approach appears to be broadly compatible with a wide range of protein-based therapies, including hormones, enzymes, and even complex cancer immunotherapies.
“This has the potential to transform the lives of patients who currently have to inject themselves daily,” said Mrsny, “such as children who need to take growth hormones.”
A Potential Revolution in Chronic Disease Management
Imagine managing diabetes with a morning pill instead of an insulin injection. Picture a world where cancer patients don’t need to visit a clinic for infusions but can instead take their immunotherapy at home. The implications are profound—not just for individual convenience and comfort, but for healthcare systems globally.
Reducing reliance on injectable drugs could lower costs, improve adherence to treatment plans, and reduce complications from improper injection technique or contamination. For vulnerable populations, especially those in developing countries or those without access to consistent medical care, such a pill could make critical treatments far more accessible.
There’s also a psychological benefit. Needle phobia affects up to one in four adults, and for many, it can be severe enough to deter them from seeking or continuing treatment. By eliminating the needle, this innovation could dramatically improve patient compliance—and outcomes.
What Are the Limits?
Despite the excitement, it’s important to remember that the technology is still in its early stages. Not all protein-based drugs may be compatible with this delivery method, especially those requiring very high doses or those that are extremely sensitive to slight changes in formulation.
Additionally, while the carrier molecule used is non-toxic, it is derived from a bacterium linked to cholera. Ensuring that it remains entirely safe, non-inflammatory, and non-immunogenic in long-term use will be a key part of human trials.
There’s also the question of manufacturing scalability, storage conditions, and cost-effectiveness at commercial levels—all factors that could influence how quickly this technology reaches pharmacies and clinics.
The Beginning of the End for Injections?
Still, there is reason for real optimism. This technology is not just a new type of pill; it’s the unlocking of an entirely new class of oral medicines. It represents a marriage of biology and biotechnology, where knowledge of natural microbial processes is harnessed to serve human health.
It’s a reminder of how some of the most groundbreaking medical innovations don’t come from high-tech labs but from paying close attention to the natural world. A bacterium’s evolutionary trick—once a mechanism for disease—could soon become a vehicle for healing.
If successful in humans, this innovation from the University of Bath may someday eliminate the needle from millions of daily routines. And for those living with chronic illness, that future couldn’t come soon enough.
More information: Alistair Taverner et al, Human Fc CH2 domain modifies cholix transcytosis pathway to facilitate efficient oral therapeutic protein delivery, Journal of Controlled Release (2025). DOI: 10.1016/j.jconrel.2025.113964