In a groundbreaking study published in Nature Cell Biology, researchers from UT Southwestern Medical Center have uncovered a surprising vulnerability in the earliest stages of brain development. They found that cells transitioning into their neural identities naturally dial down the production of ribosomes—the microscopic factories responsible for building the proteins that keep cells alive and growing. This temporary slowdown, while a normal part of development, may make these early brain cells particularly fragile when genetic mutations disrupt ribosome production even further.
The findings shed new light on why certain genetic mutations that impair ribosome formation—previously considered to have broad effects—can lead to specific neurodevelopmental disorders characterized by intellectual disability, muscle weakness, sensory deficits, and microcephaly (unusually small brain size). This new understanding offers hope that targeted treatments, even before birth, might one day be possible.
Uncovering the Hidden Timing of Cellular Fragility
The study, co-led by Dr. Michael Buszczak and Dr. Jun Wu at UT Southwestern, began with a mystery that has long puzzled neuroscientists. Certain genetic mutations, particularly in a gene called AIRIM, are known to cause a suite of debilitating developmental conditions. The AIRIM gene plays a critical role in ribosome biogenesis, but until now, it was unclear how disruptions to such a fundamental cellular process could result in such specific and localized brain defects.
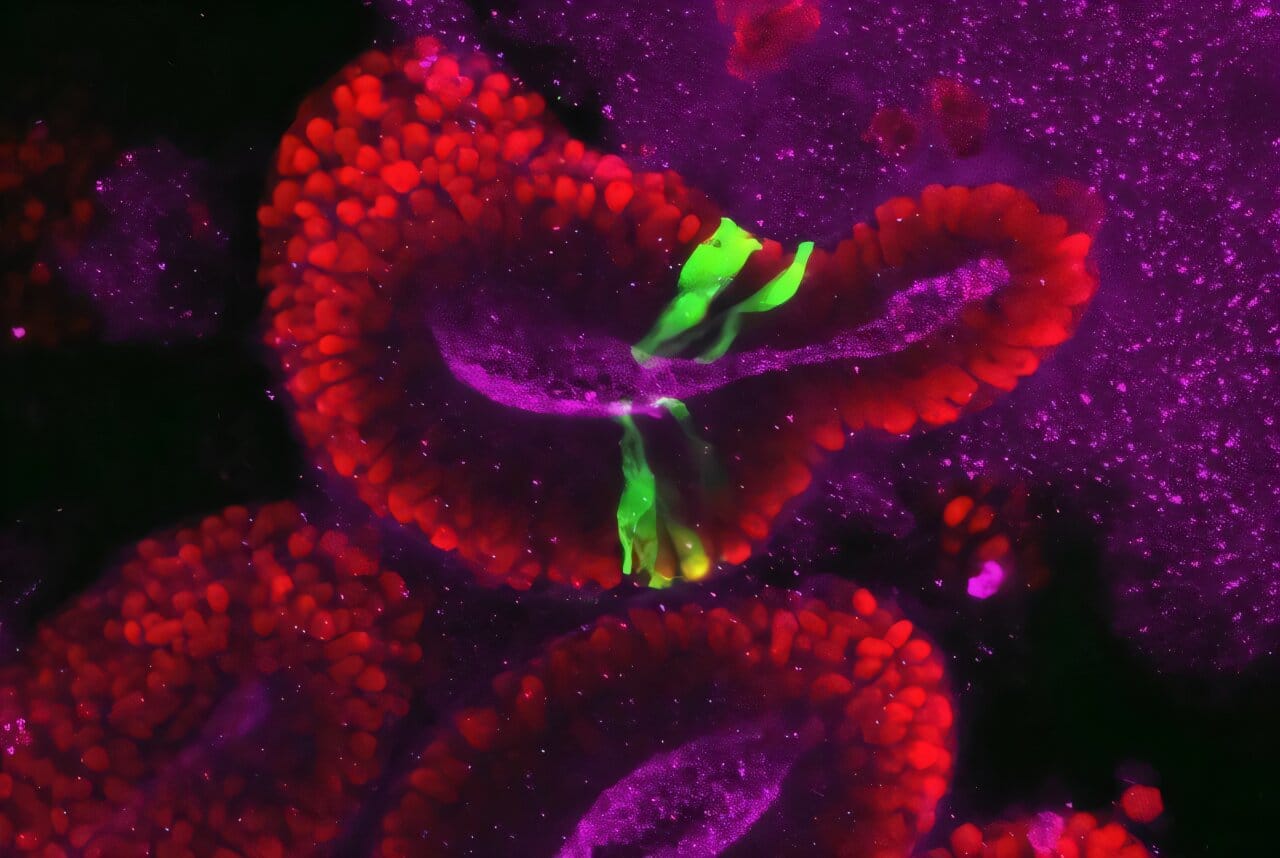
The research team used a cutting-edge model of early human brain development called a brain organoid—a miniature, lab-grown version of the human brain developed from stem cells. With help from Dr. Wu’s expertise in stem cell biology and organoid technology, they took patient-derived cells with AIRIM mutations, reprogrammed them into stem cells, and allowed them to develop into brain organoids. For comparison, they also created organoids from healthy, mutation-free cells.
As these tiny proto-brains developed, the team focused on day 15—a critical turning point in early neurodevelopment. At this stage, stem cells called neuroepithelial cells begin transforming into radial glia, the first major specialized neural cell type and the foundation for future brain structure.
It was here that the differences became stark.
Organoids with AIRIM mutations were visibly smaller and contained many dying cells. Upon closer examination, both mutant and normal organoids exhibited fewer ribosomes than earlier stages—but those with mutations had even fewer. The researchers had found their answer: the brain, during this sensitive window of development, naturally scales back protein production, and any additional disruption from mutations can tip the balance toward cellular collapse.
Why Ribosomes Matter So Much for Developing Brains
Ribosomes may be small, but they play an enormous role in cell survival and function. They translate genetic code into proteins, which carry out virtually every function in the cell. In the early brain, where rapid growth and specialization are crucial, the demand for proteins is immense.
Dr. Buszczak explained, “We find that ribosome levels decrease during neuroepithelial differentiation, a very early step in human brain development, making differentiating cells particularly vulnerable to changes in ribosome biogenesis during this time.” In other words, this stage of brain formation is a tightrope walk: cells must maintain enough protein production to survive and mature, even as their ribosome levels dip naturally.
The AIRIM mutations made this balancing act even more precarious. Cells with severely reduced ribosome production couldn’t generate enough of the proteins they needed—especially those involved in survival and differentiation—leading to cell death and organoid shrinkage.
This vulnerability, revealed for the first time, opens a new window into understanding neurodevelopmental diseases that previously seemed disconnected from ribosomal biology.
Hope Through Rescue: Restoring Protein Production
One of the most exciting findings came when the researchers asked a simple but daring question: What if we could boost ribosome activity during this fragile window?
By using genetic and pharmaceutical tools to activate mTOR, a master regulator of cell growth and protein synthesis, the team was able to significantly boost protein production in the affected cells. The result was remarkable: the mutated organoids recovered their size and produced protein levels comparable to healthy controls.
This kind of functional rescue is more than a laboratory trick—it suggests a potential therapeutic pathway for treating certain neurodevelopmental disorders in utero, before symptoms even appear. If doctors could one day identify fetuses at risk for ribosome-deficiency-related conditions, interventions that stabilize protein production could theoretically preserve brain development and prevent disability.
A Global Collaboration for a Global Challenge
The study was a major collaborative effort across multiple institutions and continents. In addition to Dr. Buszczak and Dr. Wu, the team included Dr. Chunyang Ni (now at Stanford University), Dr. Yudong Wei (postdoctoral fellow in the Buszczak Lab), and international partners Dr. Barbara Vona (University Medical Center Gottingen, Germany) and Dr. Reza Maroofian (University College London).
Together, they zeroed in on a shared group of neurodevelopmental disorders associated with AIRIM mutations—conditions marked by severe cognitive and motor impairments, hearing and vision loss, and arrested brain growth. Though rare, these disorders represent an immense burden on patients and families, and have long lacked effective treatments.
By identifying the critical moment in development when affected brain cells become most vulnerable, the study offers not just understanding, but a potential path forward.
Why This Discovery Matters Now
While the ribosome is a universal structure found in every cell, this study proves that when and where it malfunctions can make all the difference. For decades, scientists believed ribosome-related disorders would have generalized effects, given their fundamental role in biology. But this research challenges that idea and shows how timing and developmental context can shape the outcome of even the most basic cellular processes.
The broader implications go beyond neurodevelopment. If cells in other organs also experience natural drops in ribosome activity at key moments, then other ribosome-related diseases—like certain cancers or blood disorders—might also stem from similar timing mismatches. Understanding these temporal vulnerabilities could lead to new treatments across multiple fields of medicine.
A Vision for the Future: Targeting Ribosome Defects Before Birth
Looking ahead, Dr. Buszczak and his team plan to study whether other neurodevelopmental disorders caused by mutations in ribosome-related genes are also tied to similar developmental dips in protein production. If they are, it could lead to a whole new category of timing-based therapies—ones that don’t just address genetic mutations, but intervene during the windows when the brain is most sensitive to them.
That vision might still be years away, but the foundation has now been laid.
For families affected by these rare but devastating disorders, the study offers something that’s often in short supply: hope grounded in science.
And for the rest of us, it reminds us of just how finely tuned, fragile, and astonishing the process of building a human brain truly is.
More information: Chunyang Ni et al, A programmed decline in ribosome levels governs human early neurodevelopment, Nature Cell Biology (2025). DOI: 10.1038/s41556-025-01708-8