Among all forms of breast cancer, one type continues to haunt patients and researchers alike: triple-negative breast cancer. It’s a name that sounds clinical, but for those who face it, it represents fear, urgency, and often, limited hope. This aggressive subtype makes up about 15% of all breast cancer cases, yet it lacks the three key receptors—estrogen, progesterone, and HER2—that many existing therapies target. That means traditional treatments such as hormone therapy or HER2-targeted drugs simply don’t work.
For years, oncologists have relied on chemotherapy and radiation as the only options, but survival rates remain painfully low, especially for those whose cancer spreads. The medical community has long sought a breakthrough—a therapy that could finally bring precision medicine to patients with triple-negative breast cancer. Now, new research from Oregon Health & Science University (OHSU) offers a glimmer of that long-awaited hope.
The Discovery of a Powerful Molecule
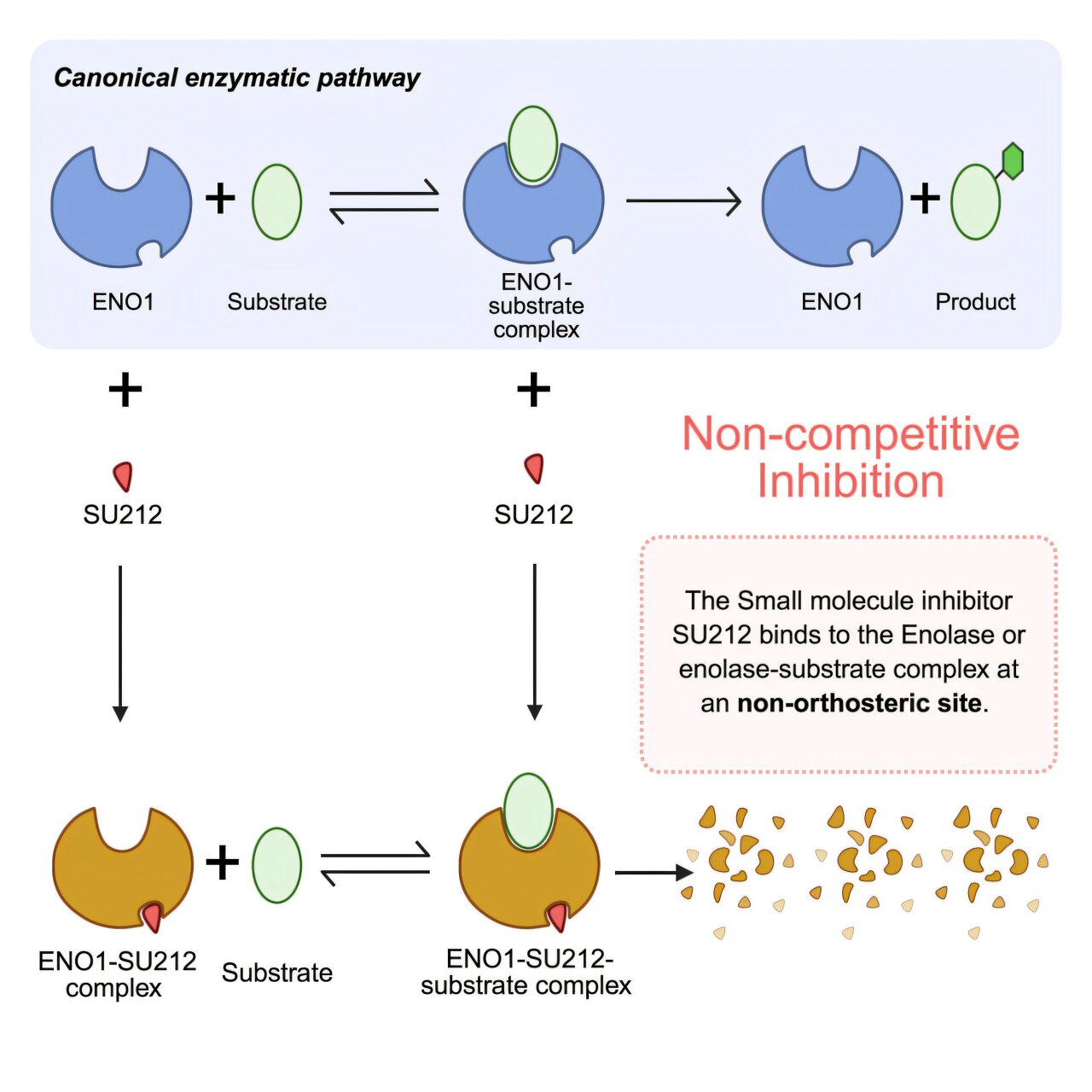
In a groundbreaking study published in Cell Reports Medicine, scientists at OHSU revealed that they have developed a molecule, known as SU212, which shows remarkable promise in combating triple-negative breast cancer. This discovery could mark a turning point in the fight against one of the most treatment-resistant forms of cancer.
The molecule works by targeting an enzyme called enolase 1 (ENO1)—a protein crucial to the way cells process energy from glucose. In normal cells, ENO1 plays a standard metabolic role, helping to convert glucose into the energy the body needs. But in cancer cells, ENO1 goes into overdrive. Its overexpression feeds the tumor’s growth, enabling cancer to thrive, spread, and resist treatment.
By binding to this enzyme, SU212 effectively disrupts cancer’s energy supply chain. Even more impressively, it induces the enzyme to degrade—essentially causing the cancer’s metabolic engine to self-destruct. In tests using a humanized mouse model of triple-negative breast cancer, SU212 successfully suppressed both tumor growth and metastasis, the process by which cancer spreads to other parts of the body.
“This is an important step forward to treat triple-negative breast cancer,” said Dr. Sanjay V. Malhotra, senior author of the study and co-director of the Center for Experimental Therapeutics at the OHSU Knight Cancer Institute. “Triple-negative breast cancer is an aggressive form of cancer, and there are no effective drugs available right now.”
From the Lab to the Clinic
While the results are early, they represent an essential proof of concept that could lead to human trials. The next step will be to advance SU212 toward clinical testing, a process that involves rigorous safety assessments, extensive funding, and the approval of the U.S. Food and Drug Administration (FDA).
Dr. Malhotra and his team are committed to moving quickly but carefully. As co-director of OHSU’s Center for Experimental Therapeutics, his mission is to translate laboratory breakthroughs into clinical applications that directly benefit patients. “There is definitely great science going on here,” he said, “and we want to translate that science for the benefit of people.”
Why This Discovery Matters
Triple-negative breast cancer (TNBC) is particularly devastating because of its unpredictable behavior and resistance to conventional therapies. Unlike other types of breast cancer, TNBC lacks the biological “handles” that drugs typically latch onto. As a result, treatment options are limited to chemotherapy—often harsh, nonspecific, and prone to failure when the disease becomes metastatic.
The discovery of SU212 introduces a new, rational approach. Instead of attacking the tumor from the outside, the molecule undermines it from within, sabotaging its metabolism at the molecular level. This strategy, known as metabolic targeting, represents one of the most exciting frontiers in modern oncology.
Moreover, SU212 could have benefits beyond triple-negative breast cancer. Because ENO1 is also implicated in other aggressive cancers—such as glioma, pancreatic cancer, and thyroid carcinoma—this molecule could form the basis for a new class of broad-spectrum cancer therapies. “A drug that targets enolase 1 could help improve the treatment of these cancers too,” Dr. Malhotra explained.
A Potential Game-Changer for Patients with Diabetes
Interestingly, the molecule’s focus on cellular metabolism may make it especially relevant for cancer patients who also suffer from metabolic disorders such as diabetes. High glucose levels in the blood can fuel certain cancers, giving them more energy to grow and spread. By suppressing ENO1, SU212 may not only slow cancer progression but also counteract this dangerous metabolic interplay.
“Because enolase 1 plays a key role in glucose regulation, SU212’s mechanism might offer dual benefits,” said Dr. Malhotra. “It could be particularly effective in cancer patients who also have metabolic diseases like diabetes.”
This insight could open a new era of personalized medicine, where treatments are designed not only around the genetic makeup of tumors but also the metabolic profiles of patients.
The Journey Behind the Discovery
Dr. Malhotra’s journey toward this breakthrough began long before his arrival at OHSU in 2020. The groundwork for SU212 was laid years earlier during his tenure at the National Cancer Institute in Bethesda, Maryland, and later refined at Stanford University, where his lab continued developing the molecule.
When he joined OHSU, he brought with him not just expertise but a vision—a desire to close the gap between scientific discovery and patient care. At OHSU’s Knight Cancer Institute, he found the perfect environment to make that vision real: a place where collaborative science thrives, and where the focus is always on delivering real-world impact.
Now, with a team of dedicated researchers, Dr. Malhotra is working to turn SU212 from a promising molecule into a life-saving medicine.
A Glimpse Into the Future
The road from discovery to treatment is long and complex, often taking years of testing and millions of dollars in investment. Yet, breakthroughs like SU212 remind us that progress is possible—and that hope can be found even in the most difficult battles.
If SU212 proves effective in human trials, it could change the way doctors approach not only triple-negative breast cancer but also a range of other metabolic-driven cancers. It would represent a shift from traditional therapies that bombard cancer cells with toxic drugs to smarter treatments that strike precisely at cancer’s internal vulnerabilities.
More information: Non-orthosteric Inhibition of Enolase 1 Impedes Growth of Triple-Negative Breast Cancer, Cell Reports Medicine (2025). DOI: 10.1016/j.xcrm.2025.102451. www.cell.com/cell-reports-medi … 2666-3791(25)00524-5